Radiation-induced CXCL16 release by breast cancer cells attracts effector T cells
- PMID: 18713980
- PMCID: PMC2587101
- DOI: 10.4049/jimmunol.181.5.3099
Radiation-induced CXCL16 release by breast cancer cells attracts effector T cells
Abstract
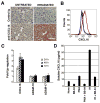
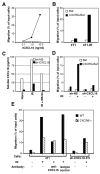
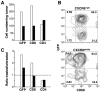
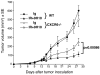
Recruitment of effector T cells to inflamed peripheral tissues is regulated by chemokines and their receptors, but the factors regulating recruitment to tumors remain largely undefined. Ionizing radiation (IR) therapy is a common treatment modality for breast and other cancers. Used as a cytocidal agent for proliferating cancer cells, IR in combination with immunotherapy has been shown to promote immune-mediated tumor destruction in preclinical studies. In this study we demonstrate that IR markedly enhanced the secretion by mouse and human breast cancer cells of CXCL16, a chemokine that binds to CXCR6 on Th1 and activated CD8 effector T cells, and plays an important role in their recruitment to sites of inflammation. Using a poorly immunogenic mouse model of breast cancer, we found that irradiation increased the migration of CD8(+)CXCR6(+) activated T cells to tumors in vitro and in vivo. CXCR6-deficient mice showed reduced infiltration of tumors by activated CD8 T cells and impaired tumor regression following treatment with local IR to the tumor and Abs blocking the negative regulator of T cell activation, CTLA-4. These results provide the first evidence that IR can induce the secretion by cancer cells of proinflammatory chemotactic factors that recruit antitumor effector T cells. The ability of IR to convert tumors into "inflamed" peripheral tissues could be exploited to overcome obstacles at the effector phase of the antitumor immune response and improve the therapeutic efficacy of immunotherapy.
Figures



References
-
- Simpson AJ, Caballero OL, Jungbluth A, Chen YT, Old LJ. Cancer/testis antigens, gametogenesis and cancer. Nat Rev Cancer. 2005;5:615–625. - PubMed
-
- Rosenberg SA, Sherry RM, Morton KE, Scharfman WJ, Yang JC, Topalian SL, Royal RE, Kammula U, Restifo NP, Hughes MS, Schwartzentruber D, Berman DM, Schwarz SL, Ngo LT, Mavroukakis SA, White DE, Steinberg SM. Tumor progression can occur despite the induction of very high levels of self/tumor antigen-specific CD8+ T cells in patients with melanoma. J Immunol. 2005;175:6169–6176. - PubMed
-
- Gajewski TF, Meng Y, Blank C, Brown I, Kacha A, Kline J, Harlin H. Immune resistance orchestrated by the tumor microenvironment. Immunol Rev. 2006;213:131–145. - PubMed
-
- Jain RK. Transport of molecules, particles, and cells in solid tumors. Annu Rev Biomed Eng. 1999;1:241–263. - PubMed
-
- Overwijk WW, Theoret MR, Finkelstein SE, Surman DR, de Jong LA, Vyth-Dreese FA, Dellemijn TA, Antony PA, Spiess PJ, Palmer DC, Heimann DM, Klebanoff CA, Yu Z, Hwang LN, Feigenbaum L, Kruisbeek AM, Rosenberg SA, Restifo NP. Tumor regression and autoimmunity after reversal of a functionally tolerant state of self-reactive CD8+ T cells. J Exp Med. 2003;198:569–580. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

