Colocalization of antigen-specific B and T cells within ectopic lymphoid tissue following immunization with exogenous antigen
- PMID: 18713997
- PMCID: PMC2769209
- DOI: 10.4049/jimmunol.181.5.3259
Colocalization of antigen-specific B and T cells within ectopic lymphoid tissue following immunization with exogenous antigen
Abstract
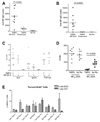
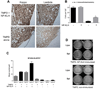
Chronic inflammation promotes the formation of ectopic lymphoid tissue morphologically resembling secondary lymphoid tissues, though it is unclear whether this is a location where Ag-specific immune responses develop or merely a site of lymphocyte accumulation. Ectopic lymphoid tissue formation is associated with many humoral autoimmune diseases, including lupus induced by tetramethylpecadentane in mice. We examined whether an immune response to 4-hydroxy-3-nitrophenyl acetyl-keyhole limpet hemocyanin (NP-KLH) and NP-OVA develops within ectopic lymphoid tissue ("lipogranulomas") induced by tetramethylpecadentane in C57BL/6 mice. Following primary immunization, NP-specific B cells bearing V186.2 and related heavy chains as well as lambda-light chains accumulated within ectopic lymphoid tissue. The number of anti-NP-secreting B cells in the ectopic lymphoid tissue was greatly enhanced by immunization with NP-KLH. Remarkably, the H chain sequences isolated from individual lipogranulomas from these mice were diverse before immunization, whereas individual lipogranulomas from single immunized mice had unique oligo- or monoclonal populations of presumptive NP-specific B cells. H chain CDR sequences bore numerous replacement mutations, consistent with an Ag-driven and T cell-mediated response. In mice adoptively transferred with OT-II or DO11 T cells, there was a striking accumulation of OVA-specific T cells in lipogranulomas after s.c. immunization with NP-OVA. The selective colocalization of proliferating, Ag-specific T and B lymphocytes in lipogranulomas from tetramethylpecadentane-treated mice undergoing primary immunization implicates ectopic lymphoid tissue as a site where Ag-specific humoral immune responses can develop. This has implications for understanding the strong association of humoral autoimmunity with lymphoid neogenesis, which may be associated with deficient censoring of autoreactive cells.
Figures




References
-
- Drayton DL, Liao S, Mounzer RH, Ruddle NH. Lymphoid organ development: from ontogeny to neogenesis. Nat.Immunol. 2006;7:344–353. - PubMed
-
- Hjelmstrom P. Lymphoid neogenesis: de novo formation of lymphoid tissue in chronic inflammation through expression of homing chemokines. J.Leukoc.Biol. 2001;69:331–339. - PubMed
-
- Cassese G, Lindenau S, de Boer B, Arce S, Hauser A, Riemekasten G, Berek C, Hiepe F, Krenn V, Radbruch A, Manz RA. Inflamed kidneys of NZB / W mice are a major site for the homeostasis of plasma cells. Eur.J.Immunol. 2001;31:2726–2732. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

