The major bactericidal activity of human seminal plasma is zinc-dependent and derived from fragmentation of the semenogelins
- PMID: 18714013
- PMCID: PMC2585754
- DOI: 10.4049/jimmunol.181.5.3413
The major bactericidal activity of human seminal plasma is zinc-dependent and derived from fragmentation of the semenogelins
Abstract
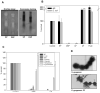
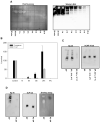
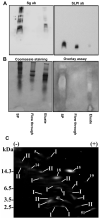
One of the major roles of seminal plasma is to provide antimicrobial protection for the spermatozoa in the female reproductive tract. We found that the bactericidal activity of seminal plasma was highest after resolution of the seminal clot and that this antibacterial activity subsequently became greatly diminished. The antibacterial activity was derived from peptides generated by fragmentation of the semenogelins while the semenogelin holoproteins displayed no antibacterial activity. After ejaculation the semenogelin-derived peptides were fragmented to smaller and smaller fragments over time and thereby lost antibacterial activity. This paralleled the loss of antibacterial activity of whole seminal plasma both in vitro and after sexual intercourse. Moreover, the antibacterial activity of the semenogelin-derived peptides generated in seminal plasma was strictly zinc-dependent both at neutral and low pH. These data provide novel roles for the resolution of seminal clots and for the high zinc concentration in human seminal plasma.
Figures





Similar articles
-
[Isolation of low-molecular-mass antibacterial mixtures from human seminal plasma].Sichuan Da Xue Xue Bao Yi Xue Ban. 2008 Sep;39(5):842-6. Sichuan Da Xue Xue Bao Yi Xue Ban. 2008. PMID: 19024329 Chinese.
-
Identification of novel semenogelin I-derived antimicrobial peptide from liquefied human seminal plasma.Peptides. 2008 Apr;29(4):505-11. doi: 10.1016/j.peptides.2008.01.009. Epub 2008 Jan 20. Peptides. 2008. PMID: 18314226
-
The GPI-anchored CD52 antigen of the sperm surface interacts with semenogelin and participates in clot formation and liquefaction of human semen.Mol Reprod Dev. 2008 Feb;75(2):326-35. doi: 10.1002/mrd.20738. Mol Reprod Dev. 2008. PMID: 17624925
-
[Activities of the fragments of semenogelin I: an update].Zhonghua Nan Ke Xue. 2013 Feb;19(2):169-72. Zhonghua Nan Ke Xue. 2013. PMID: 23441461 Review. Chinese.
-
Semenogelin I: a coagulum forming, multifunctional seminal vesicle protein.Cell Mol Life Sci. 1999 Jun;55(6-7):944-60. doi: 10.1007/s000180050346. Cell Mol Life Sci. 1999. PMID: 10412373 Free PMC article. Review.
Cited by
-
Revealing the Hidden Diagnostic Clues of Male Infertility from Human Seminal Plasma by Dispersive Solid Phase Extraction and MALDI-TOF MS.Int J Mol Sci. 2022 Sep 15;23(18):10786. doi: 10.3390/ijms231810786. Int J Mol Sci. 2022. PMID: 36142695 Free PMC article.
-
Mechanism of semen liquefaction and its potential for a novel non-hormonal contraception†.Biol Reprod. 2020 Aug 4;103(2):411-426. doi: 10.1093/biolre/ioaa075. Biol Reprod. 2020. PMID: 32529252 Free PMC article. Review.
-
Characterization of the prostate-specific antigen (PSA) catalytic mechanism: a pre-steady-state and steady-state study.PLoS One. 2014 Jul 28;9(7):e102470. doi: 10.1371/journal.pone.0102470. eCollection 2014. PLoS One. 2014. PMID: 25068395 Free PMC article.
-
Peptides released by physiological cleavage of semen coagulum proteins form amyloids that enhance HIV infection.Cell Host Microbe. 2011 Dec 15;10(6):541-50. doi: 10.1016/j.chom.2011.10.010. Cell Host Microbe. 2011. PMID: 22177559 Free PMC article.
-
Investigation of galectin-3 function in the reproductive tract by identification of binding ligands in human seminal plasma.Am J Reprod Immunol. 2014 Oct;72(4):403-12. doi: 10.1111/aji.12273. Epub 2014 May 23. Am J Reprod Immunol. 2014. PMID: 24863808 Free PMC article.
References
-
- Owen DH, Katz DF. A review of the physical and chemical properties of human semen and the formulation of a semen simulant. J. Androl. 2005;26:459–469. - PubMed
-
- De Lamirande E, Yoshida K, Yoshiike M, Iwamoto T, Gagnon C. Semenogelin, the main protein of semen coagulum, inhibits human sperm capacitation by interfering with the superoxide anion generated during this process. J. Androl. 2001;22:672–679. - PubMed
-
- Lilja H, Laurell CB. The Predominant Protein in Human Seminal Coagulate. Scand. J. Clin. Lab. Invest. 1985;45:635–641. - PubMed
-
- Malm J, Hellman J, Magnusson H, Laurell CB, Lilja H. Isolation and characterization of the major gel proteins in human semen, semenogelin I and semenogelin II. Eur. J. Biochem. 1996;238:48–53. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

