Lipopolysaccharide is a direct agonist for platelet RNA splicing
- PMID: 18714022
- PMCID: PMC2551315
- DOI: 10.4049/jimmunol.181.5.3495
Lipopolysaccharide is a direct agonist for platelet RNA splicing
Abstract
Platelets express TLR4 receptors, but its ligand LPS does not directly activate thrombotic functions nor, obviously, transcription by these anucleate cells. Platelets, however, store information that changes their phenotype over a few hours in the form of unprocessed RNA transcripts. We show even low concentrations of LPS in the presence of soluble CD14 initiated splicing of unprocessed IL-1beta RNA, with translation and accumulation of IL-1beta protein. LPS was a more robust agonist for this response than thrombin. Platelets also contained cyclooxygenase-2 pre-mRNA, which also was spliced and translated after LPS stimulation. Flow cytometry and immunocytochemistry of platelets extensively purified by negative immunodepletion showed platelets contained IL-1beta, and quantitative assessment of white blood cell contamination by CD14 real time PCR confirms that leukocytes were not the IL-1beta source, nor were they required for platelet stimulation. LPS did not initiate rapid platelet responses, but over time did prime platelet aggregation to soluble agonists, induced actin rearrangement, and initiated granule secretion with P-selectin expression that resulted the coating of quiescent leukocytes with activated platelets. LPS is a direct agonist for platelets that allows these cells to directly participate in the innate immune response to bacteria.
Figures

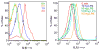

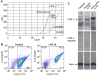


References
-
- Andonegui G, Kerfoot SM, McNagny K, Ebbert KV, Patel KD, Kubes P. Platelets express functional Toll-like receptor-4. Blood. 2005;106:2417–2423. - PubMed
-
- Cognasse F, Hamzeh H, Chavarin P, Acquart S, Genin C, Garraud O. Evidence of Toll-like receptor molecules on human platelets. Immunol Cell Biol. 2005;83:196–198. - PubMed
-
- Aslam R, Speck ER, Kim M, Crow AR, Bang KW, Nestel FP, Ni H, Lazarus AH, Freedman J, Semple JW. Platelet Toll-like receptor expression modulates lipopolysaccharide-induced thrombocytopenia and tumor necrosis factor-alpha production in vivo. Blood. 2006;107:637–641. - PubMed
-
- Stahl AL, Svensson M, Morgelin M, Svanborg C, Tarr PI, Mooney JC, Watkins SL, Johnson R, Karpman D. Lipopolysaccharide from enterohemorrhagic Escherichia coli binds to platelets through TLR4 and CD62 and is detected on circulating platelets in patients with hemolytic uremic syndrome. Blood. 2006;108:167–176. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

