Absolute quantitation of intracellular metabolite concentrations by an isotope ratio-based approach
- PMID: 18714298
- PMCID: PMC2710577
- DOI: 10.1038/nprot.2008.107
Absolute quantitation of intracellular metabolite concentrations by an isotope ratio-based approach
Abstract
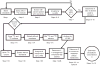
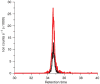
This protocol provides a method for quantitating the intracellular concentrations of endogenous metabolites in cultured cells. The cells are grown in stable isotope-labeled media to near-complete isotopic enrichment and then extracted in organic solvent containing unlabeled internal standards in known concentrations. The ratio of endogenous metabolite to internal standard in the extract is determined using mass spectrometry (MS). The product of this ratio and the unlabeled standard amount equals the amount of endogenous metabolite present in the cells. The cellular concentration of the metabolite can then be calculated on the basis of intracellular volume of the extracted cells. The protocol is exemplified using Escherichia coli and primary human fibroblasts fed uniformly with (13)C-labeled carbon sources, with detection of (13)C-assimilation by liquid chromatography-tandem MS. It enables absolute quantitation of several dozen metabolites over approximately 1 week of work.
Figures

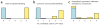



References
-
- Rabinowitz JD, Kimball E. Acidic acetonitrile for cellular metabolome extraction from Escherichia coli. Anal Chem. 2007;79:6167–6173. - PubMed
-
- Yuan J, Fowler WU, Kimball E, Lu W, Rabinowitz JD. Kinetic flux profiling of nitrogen assimilation in Escherichia coli. Nat Chem Biol. 2006;2:529–530. - PubMed
-
- Lu W, Kimball E, Rabinowitz JD. A high-performance liquid chromatography-tandem mass spectrometry method for quantitation of nitrogen-containing intracellular metabolites. J Am Soc Mass Spectrom. 2006;17:37–50. - PubMed
-
- Wu L, et al. Quantitative analysis of the microbial metabolome by isotope dilution mass spectrometry using uniformly 13C-labeled cell extracts as internal standards. Anal Biochem. 2005;336:164–171. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

