Expanded repertoire of AAV vector serotypes mediate unique patterns of transduction in mouse brain
- PMID: 18714307
- PMCID: PMC3056207
- DOI: 10.1038/mt.2008.166
Expanded repertoire of AAV vector serotypes mediate unique patterns of transduction in mouse brain
Abstract
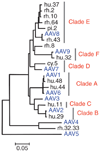
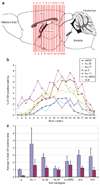
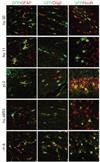
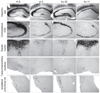
A wide diversity of adeno-associated virus (AAV) structural proteins uncovered from latent genomes in primate tissue has expanded the number of AAV vector serotypes, which can potentially confer unique cell tropism to the vector. We evaluated 17 of these vectors in the mouse brain using green fluorescent protein (GFP) as a reporter gene. A rapid initial evaluation was performed by neonatal lateral ventricle injections. Vectors made with capsids hu.32, hu.37, pi.2, hu.11, rh.8, hu.48R3, and AAV9 for comparison were selected for further analysis based on their ability to transduce large numbers of cells and result in novel patterns of cell transduction. These vectors were injected into adult brains in four major structures (cortex, striatum, hippocampus, and thalamus), and all were found to transduce neurons. In addition, hu.32, hu.11, pi.2, hu.48R3, and rh.8 resulted in GFP expression in some astrocytes or oligodendrocytes. AAVs rh.8, pi.2, hu.32, and hu.11 also appeared to result in neuronal transport of the vector genome. Vector transport was studied by a single unilateral injection into the hippocampus and vector genome was found in projection sites of the hippocampus. These unique patterns of cell transduction expand the potential repertoire for targeting AAV vectors to selected subsets of brain cells.
Figures


References
-
- Passini MA, Watson DJ, Vite CH, Landsburg DJ, Feigenbaum AL, Wolfe JH. Intraventricular brain injection of adeno-associated virus type 1 (AAV1) in neonatal mice results in complementary patterns of neuronal transduction to AAV2 and total long-term correction of storage lesions in the brains of beta-glucuronidase-deficient mice. J Virol. 2003;77:7034–7040. - PMC - PubMed
-
- Burger C, Gorbatyuk OS, Velardo MJ, Peden CS, Williams P, Zolotukhin S, et al. Recombinant AAV viral vectors pseudotyped with viral capsids from serotypes 1, 2, and 5 display differential efficiency and cell tropism after delivery to different regions of the central nervous system. Mol Ther. 2004;10:302–317. - PubMed
-
- Cearley CN, Wolfe JH. Transduction characteristics of adeno-associated virus vectors expressing cap serotypes 7, 8, 9, and Rh10 in the mouse brain. Mol Ther. 2006;13:528–537. - PubMed
-
- Taymans JM, Vandenberghe LH, Haute CV, Thiry I, Deroose CM, Mortelmans L, et al. Comparative analysis of adeno-associated viral vector serotypes 1, 2, 5, 7, and 8 in mouse brain. Hum Gene Ther. 2007;18:195–206. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

