Neutralization of botulinum neurotoxin by a human monoclonal antibody specific for the catalytic light chain
- PMID: 18714390
- PMCID: PMC2515629
- DOI: 10.1371/journal.pone.0003023
Neutralization of botulinum neurotoxin by a human monoclonal antibody specific for the catalytic light chain
Abstract
Background: Botulinum neurotoxins (BoNT) are a family of category A select bioterror agents and the most potent biological toxins known. Cloned antibody therapeutics hold considerable promise as BoNT therapeutics, but the therapeutic utility of antibodies that bind the BoNT light chain domain (LC), a metalloprotease that functions in the cytosol of cholinergic neurons, has not been thoroughly explored.
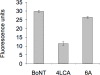
Methods and findings: We used an optimized hybridoma method to clone a fully human antibody specific for the LC of serotype A BoNT (BoNT/A). The 4LCA antibody demonstrated potent in vivo neutralization when administered alone and collaborated with an antibody specific for the HC. In Neuro-2a neuroblastoma cells, the 4LCA antibody prevented the cleavage of the BoNT/A proteolytic target, SNAP-25. Unlike an antibody specific for the HC, the 4LCA antibody did not block entry of BoNT/A into cultured cells. Instead, it was taken up into synaptic vesicles along with BoNT/A. The 4LCA antibody also directly inhibited BoNT/A catalytic activity in vitro.
Conclusions: An antibody specific for the BoNT/A LC can potently inhibit BoNT/A in vivo and in vitro, using mechanisms not previously associated with BoNT-neutralizing antibodies. Antibodies specific for BoNT LC may be valuable components of an antibody antidote for BoNT exposure.
Conflict of interest statement
Figures



References
-
- Arnon SS, Schechter R, Inglesby TV, Henderson DA, Bartlett JG, et al. Botulinum toxin as a biological weapon: medical and public health management. JAMA. 2001;285:1059–1070. - PubMed
-
- Simpson LL. Identification of the major steps in botulinum toxin action. Annu Rev Pharmacol Toxicol. 2004;44:167–193. - PubMed
-
- Dong M, Yeh F, Tepp WH, Dean C, Johnson EA, et al. SV2 is the protein receptor for botulinum neurotoxin A. Science. 2006;312:592–596. - PubMed
-
- Mahrhold S, Rummel A, Bigalke H, Davletov B, Binz T. The synaptic vesicle protein 2C mediates the uptake of botulinum neurotoxin A into phrenic nerves. FEBS Lett. 2006;580:2011–2014. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

