Clusterin interacts with Paclitaxel and confer Paclitaxel resistance in ovarian cancer
- PMID: 18714397
- PMCID: PMC2517641
- DOI: 10.1593/neo.08604
Clusterin interacts with Paclitaxel and confer Paclitaxel resistance in ovarian cancer
Abstract
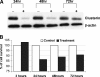
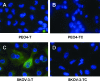
Optimal debulking followed by chemotherapy is the standard treatment of managing late-stage ovarian cancer, but chemoresistance is still a major problem. In this study, we compared expression profiles of primary tumor tissue from five long-term (>8 years) and five short-term (<2 years) ovarian cancer survivors and identified clusterin as one of the genes that were significantly up-regulated in short-term survivors. We then evaluated the prognostic significance of clusterin and its possible correlation with chemoresistance in ovarian cancer by immunohistostaining of clusterin in 62 tumor samples from patients with stage III, high-grade serous ovarian cancer. After adjusting for debulking status and age, Cox regression analyses showed that high levels of clusterin expression correlate with poor survival (hazard ratio, 1.07; 95% confidence interval, 1.002-1.443; P = .04). We also investigated clusterin in paclitaxel resistance by modulating the endogenous clusterin expression in ovarian cancer cells and treating the cells with purified clusterin. Results indicate that high-clusterin-expressing ovarian cancer cells are more resistant to paclitaxel. Moreover, exposing ovarian cancer cells to exogenous clusterin increases cells' resistance to paclitaxel. Finally, using size exclusion chromatography and fluorescently labeled paclitaxel, we demonstrated that clusterin binds to paclitaxel. In summary, our findings suggest that high levels of clusterin expression increase paclitaxel resistance in ovarian cancer cells by physically binding to paclitaxel, which may prevent paclitaxel from interacting with microtubules to induce apoptosis. Thus, clusterin is a potential therapeutic target for enhancing chemoresponsiveness in patients with a high-level clusterin expression.
Figures




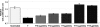


References
-
- Boring CC, Squires TS, Tong T, Montgomery S. Cancer statistics, 1994. CA Cancer J Clin. 1994;44:7–26. - PubMed
-
- Landis SH, Murray T, Bolden S, Wingo PA. Cancer statistics, 1998. CA Cancer J Clin. 1998;48:6–29. - PubMed
-
- Omura GA, Brady MF, Homesley HD, Yordan E, Major FJ, Buchsbaum HJ, Park RC. Long-term follow-up and prognostic factor analysis in advanced ovarian carcinoma: the Gynecologic Oncology Group experience. J Clin Oncol. 1991;9:1138–1150. - PubMed
-
- Barlund M, Forozan F, Kononen J, Bubendorf L, Chen Y, Bittner ML, Torhorst J, Haas P, Bucher C, Sauter G, et al. Detecting activation of ribosomal protein S6 kinase by complementary DNA and tissue microarray analysis. J Natl Cancer Inst. 2000;92:1252–1259. - PubMed
-
- Wang T, Hopkins D, Schmidt C, Silva S, Houghton R, Takita H, Repasky E, Reed SG. Identification of genes differentially over-expressed in lung squamous cell carcinoma using combination of cDNA subtraction and microarray analysis. Oncogene. 2000;19:1519–1528. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
