Two faces of chondroitin sulfate proteoglycan in spinal cord repair: a role in microglia/macrophage activation
- PMID: 18715114
- PMCID: PMC2517615
- DOI: 10.1371/journal.pmed.0050171
Two faces of chondroitin sulfate proteoglycan in spinal cord repair: a role in microglia/macrophage activation
Abstract
Background: Chondroitin sulfate proteoglycan (CSPG) is a major component of the glial scar. It is considered to be a major obstacle for central nervous system (CNS) recovery after injury, especially in light of its well-known activity in limiting axonal growth. Therefore, its degradation has become a key therapeutic goal in the field of CNS regeneration. Yet, the abundant de novo synthesis of CSPG in response to CNS injury is puzzling. This apparent dichotomy led us to hypothesize that CSPG plays a beneficial role in the repair process, which might have been previously overlooked because of nonoptimal regulation of its levels. This hypothesis is tested in the present study.
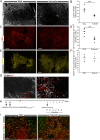
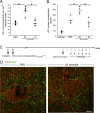
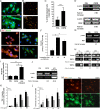
Methods and findings: We inflicted spinal cord injury in adult mice and examined the effects of CSPG on the recovery process. We used xyloside to inhibit CSPG formation at different time points after the injury and analyzed the phenotype acquired by the microglia/macrophages in the lesion site. To distinguish between the resident microglia and infiltrating monocytes, we used chimeric mice whose bone marrow-derived myeloid cells expressed GFP. We found that CSPG plays a key role during the acute recovery stage after spinal cord injury in mice. Inhibition of CSPG synthesis immediately after injury impaired functional motor recovery and increased tissue loss. Using the chimeric mice we found that the immediate inhibition of CSPG production caused a dramatic effect on the spatial organization of the infiltrating myeloid cells around the lesion site, decreased insulin-like growth factor 1 (IGF-1) production by microglia/macrophages, and increased tumor necrosis factor alpha (TNF-alpha) levels. In contrast, delayed inhibition, allowing CSPG synthesis during the first 2 d following injury, with subsequent inhibition, improved recovery. Using in vitro studies, we showed that CSPG directly activated microglia/macrophages via the CD44 receptor and modulated neurotrophic factor secretion by these cells.
Conclusions: Our results show that CSPG plays a pivotal role in the repair of injured spinal cord and in the recovery of motor function during the acute phase after the injury; CSPG spatially and temporally controls activity of infiltrating blood-borne monocytes and resident microglia. The distinction made in this study between the beneficial role of CSPG during the acute stage and its deleterious effect at later stages emphasizes the need to retain the endogenous potential of this molecule in repair by controlling its levels at different stages of post-injury repair.
Conflict of interest statement
Figures



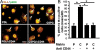
References
-
- Silver J, Miller JH. Regeneration beyond the glial scar. Nat Rev Neurosci. 2004;5:146–156. - PubMed
-
- Buss A, Pech K, Kakulas BA, Martin D, Schoenen J, et al. Growth-modulating molecules are associated with invading Schwann cells and not astrocytes in human traumatic spinal cord injury. Brain. 2007;130:940–953. - PubMed
-
- Fitch MT, Silver J. Activated macrophages and the blood-brain barrier: inflammation after CNS injury leads to increases in putative inhibitory molecules. Exp Neurol. 1997;148:587–603. - PubMed
-
- Fournier AE, Strittmatter SM. Repulsive factors and axon regeneration in the CNS. Curr Opin Neurobiol. 2001;11:89–94. - PubMed
-
- Popovich PG, Guan Z, Wei P, Huitinga I, van Rooijen N, et al. Depletion of hematogenous macrophages promotes partial hindlimb recovery and neuroanatomical repair after experimental spinal cord injury. Exp Neurol. 1999;158:351–365. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

