Improved glycemic control in intensively treated adult subjects with type 1 diabetes using insulin guidance software
- PMID: 18715213
- PMCID: PMC2979335
- DOI: 10.1089/dia.2007.0303
Improved glycemic control in intensively treated adult subjects with type 1 diabetes using insulin guidance software
Abstract
Background: Management of type 1 diabetes could be significantly improved with the availability of computerized insulin algorithms for home use.
Methods: This was a 1-year open label randomized control trial involving 123 adult subjects with type 1 diabetes (hemoglobin A1c values 7.5-11%) assigned to either the insulin guidance software (ACCU-CHEK) [Roche, Indianapolis, IN] Advisor) for personal data assistant (experimental group) or the control group. The primary aim of the study was to see if subjects using insulin dosing advisor software will improve glucose control over 1 year. The principal end point was an improvement in A1c at 6 and 12 months by >or=0.4%.
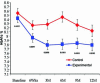
Results: Baseline demographics were similar in the two groups. Mean A1c was 8.54+/-0.11% in the control group and 8.42+/-0.11% (P=0.4265) in the experimental group. The mean A1c was significantly lower from 3 to 12 months in the experimental group (P<0.02). A1c reduction of >or=0.6% was maintained at 12 months in the experimental group. Also, a significantly higher number of subjects achieved A1c <7.5% in the experimental group from 3 to 9 months. Within target range glycemia (70-150 mg/dL) was higher in the experimental group at 3-9 months without any change in insulin dose or weight. Above target range glycemia was lower in the experimental group throughout the study. Frequency of testing per day was higher in the experimental group. Nocturnal hypoglycemia was not different between groups; however, the experimental group experienced more severe hypoglycemic events.
Conclusions: This is the first report that shows improved glycemic control can be maintained over 12 months in patients with type 1 diabetes by using Advisor with no change in insulin dose and weight.
Figures


References
-
- Diabetes Control and Complications Trial Research Group. The effect of intensive treatment of diabetes on the development, progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med. 1993;329:977–986. - PubMed
-
- Nathan DM. Long-term complications of diabetes mellitus. N Engl J Med. 1993;328:1676–1685. - PubMed
-
- Turner RC. Cull CA. Frighi V. Holman RR the UK Prospective Diabetes Study (UKPDS Group) Glycaemic control with diet, sulfonylurea, metformin, or insulin in patients with type 2 diabetes mellitus. JAMA. 1998;281:2005–2012. - PubMed
-
- UK Prospective Diabetes Study (UKPDS) Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33) Lancet. 1998;352:837–853. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

