Iris phenotypes and pigment dispersion caused by genes influencing pigmentation
- PMID: 18715234
- PMCID: PMC2862261
- DOI: 10.1111/j.1755-148X.2008.00482.x
Iris phenotypes and pigment dispersion caused by genes influencing pigmentation
Abstract
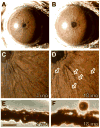
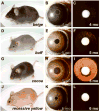
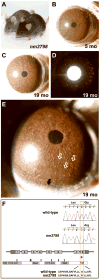
Spontaneous mutations altering mouse coat colors have been a classic resource for discovery of numerous molecular pathways. Although often overlooked, the mouse iris is also densely pigmented and easily observed, thus representing a similarly powerful opportunity for studying pigment cell biology. Here, we present an analysis of iris phenotypes among 16 mouse strains with mutations influencing melanosomes. Many of these strains exhibit biologically and medically relevant phenotypes, including pigment dispersion, a common feature of several human ocular diseases. Pigment dispersion was identified in several strains with mutant alleles known to influence melanosomes, including beige, light, and vitiligo. Pigment dispersion was also detected in the recently arising spontaneous coat color variant, nm2798. We have identified the nm2798 mutation as a missense mutation in the Dct gene, an identical re-occurrence of the slaty light mutation. These results suggest that dysregulated events of melanosomes can be potent contributors to the pigment dispersion phenotype. Combined, these findings illustrate the utility of studying iris phenotypes as a means of discovering new pathways, and re-linking old ones, to processes of pigmented cells in health and disease.
Figures



References
-
- Anderson MG, Smith RS, Hawes NL, Zabaleta A, Chang B, Wiggs JL, John SW. Mutations in genes encoding melanosomal proteins cause pigmentary glaucoma in DBA/2J mice. Nat Genet. 2002;30:81–5. - PubMed
-
- Ball SF. Pigmentary Glaucoma. In: Yanoff M, Duker JS, editors. Ophthalmology. 2. St Louis: Mosby; 2004.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

