Toll-like receptor 3 has a protective role against West Nile virus infection
- PMID: 18715906
- PMCID: PMC2573187
- DOI: 10.1128/JVI.00935-08
Toll-like receptor 3 has a protective role against West Nile virus infection
Abstract
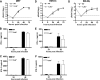
Protection against West Nile virus (WNV) infection requires rapid viral sensing and the generation of an interferon (IFN) response. Mice lacking IFN regulatory factor 3 (IRF-3) show increased vulnerability to WNV infection with enhanced viral replication and blunted IFN-stimulated gene (ISG) responses. IRF-3 functions downstream of several viral sensors, including Toll-like receptor 3 (TLR3), RIG-I, and MDA5. Cell culture studies suggest that host recognizes WNV in part, through the cytoplasmic helicase RIG-I and to a lesser extent, MDA5, both of which activate ISG expression through IRF-3. However, the role of TLR3 in vivo in recognizing viral RNA and activating antiviral defense pathways has remained controversial. We show here that an absence of TLR3 enhances WNV mortality in mice and increases viral burden in the brain. Compared to congenic wild-type controls, TLR3(-/-) mice showed relatively modest changes in peripheral viral loads. Consistent with this, little difference in multistep viral growth kinetics or IFN-alpha/beta induction was observed between wild-type and TLR3(-/-) fibroblasts, macrophages, and dendritic cells. In contrast, a deficiency of TLR3 was associated with enhanced viral replication in primary cortical neuron cultures and greater WNV infection in central nervous system neurons after intracranial inoculation. Taken together, our data suggest that TLR3 serves a protective role against WNV in part, by restricting replication in neurons.
Figures





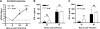
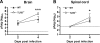

References
-
- Akira, S., S. Uematsu, and O. Takeuchi. 2006. Pathogen recognition and innate immunity. Cell 124783-801. - PubMed
-
- Alexopoulou, L., A. C. Holt, R. Medzhitov, and R. A. Flavell. 2001. Recognition of double-stranded RNA and activation of NF-κB by Toll-like receptor 3. Nature 413732-738. - PubMed
-
- Anderson, J. F., T. G. Andreadis, C. R. Vossbrinck, S. Tirrell, E. M. Wakem, R. A. French, A. E. Garmendia, and H. J. Van Kruiningen. 1999. Isolation of West Nile virus from mosquitoes, crows, and a Cooper's hawk in Connecticut. Science 2862331-2333. - PubMed
-
- Arjona, A., M. Ledizet, K. Anthony, N. Bonafe, Y. Modis, T. Town, and E. Fikrig. 2007. West Nile virus envelope protein inhibits dsRNA-induced innate immune responses. J. Immunol. 1798403-8409. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

