Natural killer cells as novel helpers in anti-herpes simplex virus immune response
- PMID: 18715907
- PMCID: PMC2573218
- DOI: 10.1128/JVI.00365-08
Natural killer cells as novel helpers in anti-herpes simplex virus immune response
Abstract
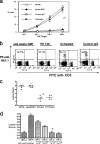
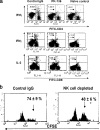
Innate defenses help to eliminate infection, but some of them also play a major role in shaping the magnitude and efficacy of the adaptive immune response. With regard to influencing subsequent adaptive immunity, NK cells aided by dendritic cells may be the most relevant components of the innate reaction to herpes simplex virus (HSV) infection. We confirm that mice lacking or depleted of NK cells are susceptible to HSV-induced lesions. The quantity and quality of CD8(+) cytotoxic T lymphocytes generated in the absence of NK cells were diminished, thereby contributing to susceptibility to HSV-induced encephalitis. We demonstrate a novel helper role for NK cells, in that NK cells compensate for the loss of CD4 helper T cells and NK cell supplementation enhances the function of wild type anti-HSV CD8 T cells. In addition, NK cells were able to partially rescue the dysfunctional CD8(+) T cells generated in the absence of CD4 T helper cells, thereby performing a novel rescue function. Hence, NK cells may well be exploited for enhancing and rescuing the T-cell response in situations where the CD4 helper response is affected.
Figures




References
-
- Arase, H., E. S. Mocarski, A. E. Campbell, A. B. Hill, and L. L. Lanier. 2002. Direct recognition of cytomegalovirus by activating and inhibitory NK cell receptors. Science 2961323-1326. - PubMed
-
- Bancroft, G. J. 1993. The role of natural killer cells in innate resistance to infection. Curr. Opin. Immunol. 5503-510. - PubMed
-
- Biron, C. A., K. S. Byron, and J. L. Sullivan. 1989. Severe herpesvirus infections in an adolescent without natural killer cells. N. Engl. J. Med. 3201731-1735. - PubMed
-
- Bloomfield, S. E., and C. Lopez. 1980. Herpes infections in the immunosuppressed host. Ophthalmology 871226-1235. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

