Enhancement of human immunodeficiency virus (HIV)-specific CD8+ T cells in cerebrospinal fluid compared to those in blood among antiretroviral therapy-naive HIV-positive subjects
- PMID: 18715919
- PMCID: PMC2573184
- DOI: 10.1128/JVI.01190-08
Enhancement of human immunodeficiency virus (HIV)-specific CD8+ T cells in cerebrospinal fluid compared to those in blood among antiretroviral therapy-naive HIV-positive subjects
Abstract
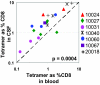
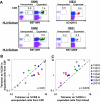
During untreated human immunodeficiency virus type 1 (HIV-1) infection, virus-specific CD8(+) T cells partially control HIV replication in peripheral lymphoid tissues, but host mechanisms of HIV control in the central nervous system (CNS) are incompletely understood. We characterized HIV-specific CD8(+) T cells in cerebrospinal fluid (CSF) and peripheral blood among seven HIV-positive antiretroviral therapy-naïve subjects. All had grossly normal brain magnetic resonance imaging and spectroscopy and normal neuropsychometric testing. Frequencies of epitope-specific CD8(+) T cells by direct tetramer staining were on average 2.4-fold higher in CSF than in blood (P = 0.0004), while HIV RNA concentrations were lower. Cells from CSF were readily expanded ex vivo and responded to a broader range of HIV-specific human leukocyte antigen class I restricted optimal peptides than did expanded cells from blood. HIV-specific CD8(+) T cells, in contrast to total CD8(+) T cells, in CSF and blood were at comparable maturation states, as assessed by CD45RO and CCR7 staining. The strong relationship between higher T-cell frequencies and lower levels of viral antigen in CSF could be the result of increased migration to and/or preferential expansion of HIV-specific T cells within the CNS. This suggests an important role for HIV-specific CD8(+) T cells in control of intrathecal viral replication.
Figures



References
-
- Addo, M. M., X. G. Yu, A. Rathod, D. Cohen, R. L. Eldridge, D. Strick, M. N. Johnston, C. Corcoran, A. G. Wurcel, C. A. Fitzpatrick, M. E. Feeney, W. R. Rodriguez, N. Basgoz, R. Draenert, D. R. Stone, C. Brander, P. J. Goulder, E. S. Rosenberg, M. Altfeld, and B. D. Walker. 2003. Comprehensive epitope analysis of human immunodeficiency virus type 1 (HIV-1)-specific T-cell responses directed against the entire expressed HIV-1 genome demonstrate broadly directed responses, but no correlation to viral load. J. Virol. 772081-2092. - PMC - PubMed
-
- Altfeld, M., J. van Lunzen, N. Frahm, X. G. Yu, C. Schneider, R. L. Eldridge, M. E. Feeney, D. Meyer-Olson, H. J. Stellbrink, and B. D. Walker. 2002. Expansion of pre-existing, lymph node-localized CD8+ T cells during supervised treatment interruptions in chronic HIV-1 infection. J. Clin. Investig. 109837-843. - PMC - PubMed
-
- Baenziger, J., H. Hengartner, R. M. Zinkernagel, and G. A. Cole. 1986. Induction or prevention of immunopathological disease by cloned cytotoxic T cell lines specific for lymphocytic choriomeningitis virus. Eur. J. Immunol. 16387-393. - PubMed
-
- Bagetta, G., M. T. Corasaniti, A. M. Paoletti, L. Berliocchi, R. Nistico, A. M. Giammarioli, W. Malorni, and A. Finazzi-Agro. 1998. HIV-1 gp120-induced apoptosis in the rat neocortex involves enhanced expression of cyclo-oxygenase type 2 (COX-2). Biochem. Biophys. Res. Commun. 244819-824. - PubMed
-
- Barks, J. D., X. H. Liu, R. Sun, and F. S. Silverstein. 1997. gp120, a human immunodeficiency virus-1 coat protein, augments excitotoxic hippocampal injury in perinatal rats. Neuroscience 76397-409. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

