Root and shoot respiration of perennial ryegrass are supplied by the same substrate pools: assessment by dynamic 13C labeling and compartmental analysis of tracer kinetics
- PMID: 18715953
- PMCID: PMC2556832
- DOI: 10.1104/pp.108.127324
Root and shoot respiration of perennial ryegrass are supplied by the same substrate pools: assessment by dynamic 13C labeling and compartmental analysis of tracer kinetics
Abstract
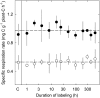
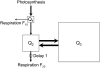
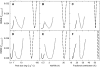
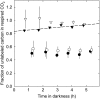
The substrate supply system for respiration of the shoot and root of perennial ryegrass (Lolium perenne) was characterized in terms of component pools and the pools' functional properties: size, half-life, and contribution to respiration of the root and shoot. These investigations were performed with perennial ryegrass growing in constant conditions with continuous light. Plants were labeled with (13)CO(2)/(12)CO(2) for periods ranging from 1 to 600 h, followed by measurements of the rates and (13)C/(12)C ratios of CO(2) respired by shoots and roots in the dark. Label appearance in roots was delayed by approximately 1 h relative to shoots; otherwise, the tracer time course was very similar in both organs. Compartmental analysis of respiratory tracer kinetics indicated that, in both organs, three pools supplied 95% of all respired carbon (a very slow pool whose kinetics could not be characterized provided the remaining 5%). The pools' half-lives and relative sizes were also nearly identical in shoot and root (half-life < 15 min, approximately 3 h, and 33 h). An important role of short-term storage in supplying respiration was apparent in both organs: only 43% of respiration was supplied by current photosynthate (fixed carbon transferred directly to centers of respiration via the two fastest pools). The residence time of carbon in the respiratory supply system was practically the same in shoot and root. From this and other evidence, we argue that both organs were supplied by the same pools and that the residence time was controlled by the shoot via current photosynthate and storage deposition/mobilization fluxes.
Figures

References
-
- Amthor JS (1989) Respiration and Crop Productivity. Springer Verlag, New York
-
- ap Rees T (1980) Assessment of the contributions of metabolic pathways to plant respiration. In DD Davies, ed, The Biochemistry of Plants: A Comprehensive Treatise, Vol 2. Academic Press, San Diego, pp 1–29
-
- Atkins GL (1969) Multicompartment Models in Biological Systems. Methuen, London
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

