Ischemia-induced exocytosis of Weibel-Palade bodies mobilizes stem cells
- PMID: 18715993
- PMCID: PMC2588100
- DOI: 10.1681/ASN.2007111200
Ischemia-induced exocytosis of Weibel-Palade bodies mobilizes stem cells
Abstract
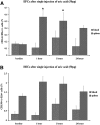
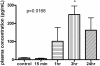
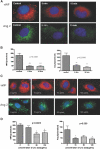
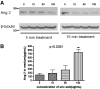
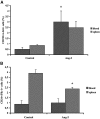
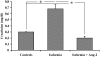
Recruitment of various stem and progenitor cells is crucial for the regeneration of an injured organ. Levels of uric acid, one of the prototypical "alarm signals," surge after ischemia-reperfusion injury. Exogenous uric acid rapidly mobilizes endothelial progenitor cells and hematopoietic stem cells and protects the kidney from ischemia. The relatively fast responses to uric acid suggest that preformed second messengers may be released from a storage pool. Here, it is reported that monosodium urate (MSU) results in exocytosis of Weibel-Palade bodies in vitro and in vivo, leading to the release of IL-8, von Willebrand factor, and angiopoietin 2 in the culture medium or circulation. Confocal and immunoelectron microscopy confirmed depletion of von Willebrand factor in MSU-treated aortic endothelial cells. Angiopoietin 2 alone induced exocytosis of Weibel-Palade bodies, mobilized hematopoietic stem cells and depleted splenic endothelial progenitor cells, partially reproducing the actions of MSU. In addition, pretreatment with angiopoietin 2 protected the kidneys from an ischemic insult, suggesting that the previously reported renoprotection conferred by MSU likely results from exocytosis of Weibel-Palade bodies. Furthermore, experiments with toll-like receptor 4 (TLR-4)-and TLR-2-deficient mice demonstrated that uric acid-induced exocytosis of Weibel-Palade bodies is mediated by TLR-4 and that uric acid-induced release of IL-8 requires both TLR-2 and TLR-4. In summary, these results suggest that exocytosis of Weibel-Palade bodies links postischemic repair with inflammation and mobilization of stem cells.
Figures





References
-
- Takahashi T, Kalka C, Masuda H, Chen D, Silver M, Kearney M, Magner M, Isner JM, Asahara T: Ischemia- and cytokine-induced mobilization of bone marrow-derived endothelial progenitor cells for neovascularization. Nat Med 5: 434–438, 1999 - PubMed
-
- Shintani S, Murohara T, Ikeda H, Ueno T, Honma T, Katoh A, Sasaki K, Shimada T, Oike Y, Imaizumi T: Mobilization of endothelial progenitor cells in patients with acute myocardial infarction. Circulation 103: 2776–2779, 2001 - PubMed
-
- Gill M, Dias S, Hattori K, Rivera ML, Hicklin D, Witte L, Girardi L, Yurt R, Himel H, Rafii S: Vascular trauma induces rapid but transient mobilization of VEGFR2(+)AC133(+) endothelial precursor cells. Circ Res 88: 167–174, 2001 - PubMed
-
- Patschan D, Krupincza K, Patschan S, Zhang Z, Hamby C, Goligorsky MS: Dynamics of mobilization and homing of endothelial progenitor cells after acute renal ischemia: Modulation by ischemic preconditioning. Am J Physiol Renal Physiol 291: F176–F185, 2006 - PubMed
-
- Zimmet JM, Hare JM: Nitroso-redox interactions in the cardiovascular system. Circulation 114: 1531–1544, 2006 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

