Peripheral, but not central, CB1 antagonism provides food intake-independent metabolic benefits in diet-induced obese rats
- PMID: 18716045
- PMCID: PMC2570394
- DOI: 10.2337/db08-0161
Peripheral, but not central, CB1 antagonism provides food intake-independent metabolic benefits in diet-induced obese rats
Abstract
Objective: Blockade of the CB1 receptor is one of the promising strategies for the treatment of obesity. Although antagonists suppress food intake and reduce body weight, the role of central versus peripheral CB1 activation on weight loss and related metabolic parameters remains to be elucidated. We therefore specifically assessed and compared the respective potential relevance of central nervous system (CNS) versus peripheral CB1 receptors in the regulation of energy homeostasis and lipid and glucose metabolism in diet-induced obese (DIO) rats.
Research design and methods: Both lean and DIO rats were used for our experiments. The expression of key enzymes involved in lipid metabolism was measured by real-time PCR, and euglycemic-hyperinsulinemic clamps were used for insulin sensitivity and glucose metabolism studies.
Results: Specific CNS-CB1 blockade decreased body weight and food intake but, independent of those effects, had no beneficial influence on peripheral lipid and glucose metabolism. Peripheral treatment with CB1 antagonist (Rimonabant) also reduced food intake and body weight but, in addition, independently triggered lipid mobilization pathways in white adipose tissue and cellular glucose uptake. Insulin sensitivity and skeletal muscle glucose uptake were enhanced, while hepatic glucose production was decreased during peripheral infusion of the CB1 antagonist. However, these effects depended on the antagonist-elicited reduction of food intake.
Conclusions: Several relevant metabolic processes appear to independently benefit from peripheral blockade of CB1, while CNS-CB1 blockade alone predominantly affects food intake and body weight.
Figures
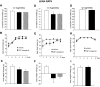
 , vehicle-pf. B, E, and H: ♦, vehicle; ▪, CB1 antagonist.
, vehicle-pf. B, E, and H: ♦, vehicle; ▪, CB1 antagonist.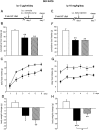
 , vehicle-pf. C and G: ♦, vehicle; ▪, CB1 antagonist.
, vehicle-pf. C and G: ♦, vehicle; ▪, CB1 antagonist.
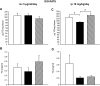
 , vehicle-pf.
, vehicle-pf.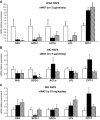
 , vehicle-pf.
, vehicle-pf.
 , vehicle-pf.
, vehicle-pf.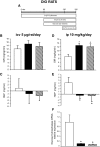
 , vehicle-pf.
, vehicle-pf.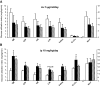
 , vehicle-pf.
, vehicle-pf.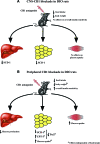
References
-
- Williams CM, Kirkham TC: Anandamide induces overeating: mediation by central cannabinoid (CB1) receptors. Psychopharmacology (Berl) 143: 315–317, 1999 - PubMed
-
- Di Marzo V, Goparaju SK, Wang L, Liu J, Batkai S, Jarai Z, Fezza F, Miura GI, Palmiter RD, Sugiura T, Kunos G: Leptin-regulated endocannabinoids are involved in maintaining food intake. Nature 410: 822–825, 2001 - PubMed
-
- Breivogel CS, Sim LJ, Childers SR: Regional differences in cannabinoid receptor/G-protein coupling in rat brain. J Pharmacol Exp Ther 282: 1632–1642, 1997 - PubMed
-
- Tsou K, Brown S, Sanudo-Pena MC, Mackie K, Walker JM: Immunohistochemical distribution of cannabinoid CB1 receptors in the rat central nervous system. Neuroscience 83: 393–411, 1998 - PubMed
-
- Van Gaal LF, Rissanen AM, Scheen AJ, Ziegler O, Rossner S: Effects of the cannabinoid-1 receptor blocker rimonabant on weight reduction and cardiovascular risk factors in overweight patients: 1-year experience from the RIO-Europe study. Lancet 365: 1389–1397, 2005 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

