A fast mode of membrane fusion dependent on tight SNARE zippering
- PMID: 18716205
- PMCID: PMC6671043
- DOI: 10.1523/JNEUROSCI.0860-08.2008
A fast mode of membrane fusion dependent on tight SNARE zippering
Abstract
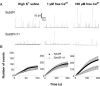
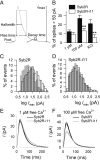
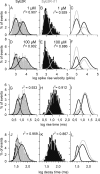
SNARE (soluble N-ethylmaleimide-sensitive factor attachment protein receptor) proteins have a key role in membrane fusion. It is commonly assumed that pairing of SNARE proteins anchored in opposing membranes overcomes the repulsion energy between membranes, thereby catalyzing fusion. In this study, we have increased the distance between the coiled-coil SNARE motif and the transmembrane domain of the vesicular SNARE synaptobrevin-2 by insertion of a flexible linker to analyze how an increased intermembrane distance affects exocytosis. Synaptobrevin-2 lengthening did not change the frequency of exocytotic events measured at 1 mum free calcium but prevented the increase in the secretory activity triggered by higher calcium concentration. Exocytotic events monitored in adrenal chromaffin cells by means of carbon fiber amperometry were classified in two groups according to the rate and extent of fusion pore expansion. Lengthening the juxtamembrane region of synaptobrevin-2 severely reduced the occurrence of rapid single events, leaving slow ones unchanged. It also impaired the increase in the fast-fusion mode that normally follows elevation of intracellular Ca2+ levels. We conclude that mild stimuli trigger slow fusion events that do not rely on a short intermembrane distance. In contrast, a short intermembrane distance mediated by tight zippering of SNAREs is essential to a component of the secretory response elicited by robust stimuli and characterized by rapid dilation of the fusion pore.
Figures

References
-
- Albillos A, Dernick G, Horstmann H, Almers W, Alvarez de Toledo G, Lindau M. The exocytotic event in chromaffin cells revealed by patch amperometry. Nature. 1997;389:509–512. - PubMed
-
- Amatore C, Bouret Y, Travis ER, Wightman RM. Interplay between membrane dynamics, diffusion and swelling pressure governs individual vesicular exocytotic events during release of adrenaline by chromaffin cells. Biochimie. 2000;82:481–496. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous
