cGMP produced by NO-sensitive guanylyl cyclase essentially contributes to inflammatory and neuropathic pain by using targets different from cGMP-dependent protein kinase I
- PMID: 18716216
- PMCID: PMC6671070
- DOI: 10.1523/JNEUROSCI.2128-08.2008
cGMP produced by NO-sensitive guanylyl cyclase essentially contributes to inflammatory and neuropathic pain by using targets different from cGMP-dependent protein kinase I
Abstract
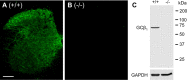
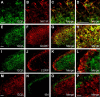
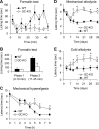
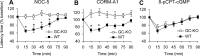
A large body of evidence indicates that the release of nitric oxide (NO) is crucial for the central sensitization of pain pathways during both inflammatory and neuropathic pain. Here, we investigated the distribution of NO-sensitive guanylyl cyclase (NO-GC) in the spinal cord and in dorsal root ganglia, and we characterized the nociceptive behavior of mice deficient in NO-GC (GC-KO mice). We show that NO-GC is distinctly expressed in neurons of the mouse dorsal horn, whereas its distribution in dorsal root ganglia is restricted to non-neuronal cells. GC-KO mice exhibited a considerably reduced nociceptive behavior in models of inflammatory or neuropathic pain, but their responses to acute pain were not impaired. Moreover, GC-KO mice failed to develop pain sensitization induced by intrathecal administration of drugs releasing NO or carbon monoxide. Surprisingly, during spinal nociceptive processing, cGMP produced by NO-GC may activate signaling pathways different from cGMP-dependent protein kinase I (cGKI), whereas cGKI can be activated by natriuretic peptide receptor-B dependent cGMP production. Together, our results provide evidence that NO-GC is crucially involved in the central sensitization of pain pathways during inflammatory and neuropathic pain.
Figures


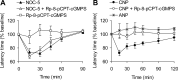
References
-
- Boettger MK, Uceyler N, Zelenka M, Schmitt A, Reif A, Chen Y, Sommer C. Differences in inflammatory pain in nNOS-, iNOS- and eNOS-deficient mice. Eur J Pain. 2007;11:810–818. - PubMed
-
- Chu YC, Guan Y, Skinner J, Raja SN, Johns RA, Tao YX. Effect of genetic knock-out or pharmacologic inhibition of neuronal nitric oxide synthase on complete Freund's adjuvant-induced persistent pain. Pain. 2005;119:113–123. - PubMed
-
- Davis KL, Martin E, Turko IV, Murad F. Novel effects of nitric oxide. Annu Rev Pharmacol Toxicol. 2001;41:203–236. - PubMed
-
- Decosterd I, Woolf CJ. Spared nerve injury: an animal model of persistent peripheral neuropathic pain. Pain. 2000;87:149–158. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous
