Genome sequence of a Lancefield group C Streptococcus zooepidemicus strain causing epidemic nephritis: new information about an old disease
- PMID: 18716664
- PMCID: PMC2516327
- DOI: 10.1371/journal.pone.0003026
Genome sequence of a Lancefield group C Streptococcus zooepidemicus strain causing epidemic nephritis: new information about an old disease
Abstract
Outbreaks of disease attributable to human error or natural causes can provide unique opportunities to gain new information about host-pathogen interactions and new leads for pathogenesis research. Poststreptococcal glomerulonephritis (PSGN), a sequela of infection with pathogenic streptococci, is a common cause of preventable kidney disease worldwide. Although PSGN usually occurs after infection with group A streptococci, organisms of Lancefield group C and G also can be responsible. Despite decades of study, the molecular pathogenesis of PSGN is poorly understood. As a first step toward gaining new information about PSGN pathogenesis, we sequenced the genome of Streptococcus equi subsp. zooepidemicus strain MGCS10565, a group C organism that caused a very large and unusually severe epidemic of nephritis in Brazil. The genome is a circular chromosome of 2,024,171 bp. The genome shares extensive gene content, including many virulence factors, with genetically related group A streptococci, but unexpectedly lacks prophages. The genome contains many apparently foreign genes interspersed around the chromosome, consistent with the presence of a full array of genes required for natural competence. An inordinately large family of genes encodes secreted extracellular collagen-like proteins with multiple integrin-binding motifs. The absence of a gene related to speB rules out the long-held belief that streptococcal pyrogenic exotoxin B or antibodies reacting with it singularly cause PSGN. Many proteins previously implicated in GAS PSGN, such as streptokinase, are either highly divergent in strain MGCS10565 or are not more closely related between these species than to orthologs present in other streptococci that do not commonly cause PSGN. Our analysis provides a comparative genomics framework for renewed appraisal of molecular events underlying APSGN pathogenesis.
Conflict of interest statement
Figures





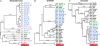

References
-
- Balter S, Benin A, Pinto SW, Teixeira LM, Alvim GG, et al. Epidemic nephritis in Nova Serrana, Brazil. Lancet. 2000;355:1776–1780. - PubMed
-
- Nicholson ML, Ferdinand L, Sampson JS, Benin A, Balter S, et al. Analysis of immunoreactivity to a Streptococcus equi subsp. zooepidemicus M-like protein To confirm an outbreak of poststreptococcal glomerulonephritis, and sequences of M-like proteins from isolates obtained from different host species. J Clin Microbiol. 2000;38:4126–4130. - PMC - PubMed
-
- Timoney JF. The pathogenic equine streptococci. Vet Res. 2004;35:397–409. - PubMed
-
- Albarracin C, Rosencrance G, Boland J, Hernandez JE. Bacteremia due to streptococcus zooepidemicus associated with an abdominal aortic aneurysm. W V Med J. 1998;94:90–92. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

