Complex ecological dynamics and eradicability of the vector borne macroparasitic disease, lymphatic filariasis
- PMID: 18716676
- PMCID: PMC2518518
- DOI: 10.1371/journal.pone.0002874
Complex ecological dynamics and eradicability of the vector borne macroparasitic disease, lymphatic filariasis
Abstract
Background: The current global efforts to control the morbidity and mortality caused by infectious diseases affecting developing countries--such as HIV/AIDS, polio, tuberculosis, malaria and the Neglected Tropical Diseases (NTDs)-have led to an increasing focus on the biological controllability or eradicability of disease transmission by management action. Here, we use an age-structured dynamical model of lymphatic filariasis transmission to show how a quantitative understanding of the dynamic processes underlying infection persistence and extinction is key to evaluating the eradicability of this macroparasitic disease.
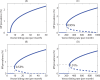
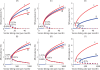
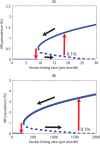
Methodology/principal findings: We investigated the persistence and extinction dynamics of lymphatic filariasis by undertaking a numerical equilibrium analysis of a deterministic model of parasite transmission, based on varying values of the initial L3 larval density in the system. The results highlighted the likely occurrence of complex dynamics in parasite transmission with three major outcomes for the eradicability of filariasis. First, both vector biting and worm breakpoint thresholds are shown to be complex dynamic entities with values dependent on the nature and magnitude of vector-and host specific density-dependent processes and the degree of host infection aggregation prevailing in endemic communities. Second, these thresholds as well as the potential size of the attractor domains and hence system resilience are strongly dependent on peculiarities of infection dynamics in different vector species. Finally, the existence of multiple stable states indicates the presence of hysteresis nonlinearity in the filariasis system dynamics in which infection thresholds for infection invasion are lower but occur at higher biting rates than do the corresponding thresholds for parasite elimination.
Conclusions/significance: The variable dynamic nature of thresholds and parasite system resilience reflecting both initial conditions and vector species-infection specificities, and the existence of hysteresis loop phenomenon, suggests that eradication of filariasis may require taking a more flexible and locally relevant approach to designing elimination programmes compared to the current command and control approach advocated by the global programme.
Conflict of interest statement
Figures



References
-
- Hotez P, Raff S, Fenwick A, Richards F, Jr, Molyneux DH. Recent progress in integrated neglected tropical disease control. Trends Parasitol. 2007;23:511–514. - PubMed
-
- Lammie PJ, Fenwick A, Utzinger J. A blueprint for success: integration of neglected tropical disease control programmes. Trends Parasitol. 2006;22:313–321. - PubMed
-
- Michael E, Malecela-Lazaro MN, Simonsen PE, Pedersen EM, Barker G, et al. Mathematical modelling and the control of lymphatic filariasis. Lancet Infect Dis. 2004;4:223–234. - PubMed
-
- Michael E, Malecela-Lazaro MN, Kabali C, Snow LC, Kazura JW. Mathematical models and lymphatic filariasis control: endpoints and optimal interventions. Trends Parasitol. 2006;22:226–233. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

