A fluorescent glycolipid-binding peptide probe traces cholesterol dependent microdomain-derived trafficking pathways
- PMID: 18716682
- PMCID: PMC2518528
- DOI: 10.1371/journal.pone.0002933
A fluorescent glycolipid-binding peptide probe traces cholesterol dependent microdomain-derived trafficking pathways
Abstract
Background: The uptake and intracellular trafficking of sphingolipids, which self-associate into plasma membrane microdomains, is associated with many pathological conditions, including viral and toxin infection, lipid storage disease, and neurodegenerative disease. However, the means available to label the trafficking pathways of sphingolipids in live cells are extremely limited. In order to address this problem, we have developed an exogenous, non-toxic probe consisting of a 25-amino acid sphingolipid binding domain, the SBD, derived from the amyloid peptide Abeta, and conjugated by a neutral linker with an organic fluorophore. The current work presents the characterization of the sphingolipid binding and live cell trafficking of this novel probe, the SBD peptide. SBD was the name given to a motif originally recognized by Fantini et al in a number of glycolipid-associated proteins, and was proposed to interact with sphingolipids in membrane microdomains.
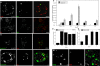
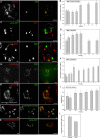
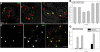
Methodology/principal findings: In accordance with Fantini's model, optimal SBD binding to membranes depends on the presence of sphingolipids and cholesterol. In synthetic membrane binding assays, SBD interacts preferentially with raft-like lipid mixtures containing sphingomyelin, cholesterol, and complex gangliosides in a pH-dependent manner, but is less glycolipid-specific than Cholera toxin B (CtxB). Using quantitative time-course colocalization in live cells, we show that the uptake and intracellular trafficking route of SBD is unlike that of either the non-raft marker Transferrin or the raft markers CtxB and Flotillin2-GFP. However, SBD traverses an endolysosomal route that partially intersects with raft-associated pathways, with a major portion being diverted at a late time point to rab11-positive recycling endosomes. Trafficking of SBD to acidified compartments is strongly disrupted by cholesterol perturbations, consistent with the regulation of sphingolipid trafficking by cholesterol.
Conclusions/significance: The current work presents the characterization and trafficking behavior of a novel sphingolipid-binding fluorescent probe, the SBD peptide. We show that SBD binding to membranes is dependent on the presence of cholesterol, sphingomyelin, and complex glycolipids. In addition, SBD targeting through the endolysosomal pathway in neurons is highly sensitive to cholesterol perturbations, making it a potentially useful tool for the analysis of sphingolipid trafficking in disease models that involve changes in cholesterol metabolism and storage.
Conflict of interest statement
Figures






References
-
- Munro S. Lipid rafts: elusive or illusive? Cell. 2003;115:377–388. - PubMed
-
- Edidin M. The state of lipid rafts: from model membranes to cells. Annu Rev Biophys Biomol Struct. 2003;32:257–283. - PubMed
-
- Brown DA, London E. Structure and function of sphingolipid- and cholesterol-rich membrane rafts. J Biol Chem. 2000;275:17221–17224. - PubMed
-
- Simons K, Ikonen E. Functional rafts in cell membranes. Nature. 1997;387:569–572. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

