A pilot study to compare tobramycin 80 mg injectable preparation with 300 mg solution for inhalation in cystic fibrosis patients
- PMID: 18716688
- PMCID: PMC2679548
- DOI: 10.1155/2008/202464
A pilot study to compare tobramycin 80 mg injectable preparation with 300 mg solution for inhalation in cystic fibrosis patients
Abstract
Background: Inhaled tobramycin has been shown to improve lung function in cystic fibrosis (CF) patients chronically infected with Pseudomonas aeruginosa. However, to date no comparative data are available for different dose regimens used in clinical practice.
Objectives: To compare the clinical efficacy of the two most commonly used treatment regimens of inhaled tobramycin in patients with CF.
Methods: In an open crossover study of CF patients, subjects were randomly allocated to receive either 80 mg tobramycin twice-daily continuous treatment or 300 mg tobramycin twice daily in cycles of 28 days on and 28 days off treatment. After three months, patients were switched to the alternative treatment regimen.
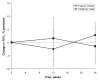
Results: A total of 32 patients with a mean (+/- SD) age of 18.5+/-8.6 years were included in the study. Compared with the treatment period using colistin, forced expiratory volume in 1 s decreased by -2.1+/-13.8% in the 80 mg tobramycin group and increased by +2.3+/-13.0% in the 300 mg group. Similar changes were observed in forced vital capacity (-2.5+/-12.9% in the 80 mg tobramycin group versus +2.5+/-9.6% in the 300 mg tobramycin group). Variability in responses was large but the differences were not statistically significant. Personal preference indicated that the majority of patients preferred the high-dose cycle compared with the lower dose continuous inhalation, but this was not linked to objective data on efficacy.
Conclusions: The present trial fails to provide convincing evidence for superiority in efficacy of either of the two treatment regimens of inhaled tobramycin in CF patients.
HISTORIQUE :: Il est démontré que la tobramycine en aérosol améliore la fonction pulmonaire chez les patients atteints de fibrose kystique (FK) et d’une infection chronique à Pseudomonas aeruginosa. Cependant, il n’existe encore aucunes données comparatives pour les diverses posologies utilisées en pratique clinique.
OBJECTIFS :: Comparer l’efficacité clinique des deux schémas thérapeutiques de tobramycine en aérosol les plus utilisés chez les patients atteints de FK.
MÉTHODOLOGIE :: Dans une étude transversale ouverte de patients atteints de FK, les sujets ont été divisés au hasard entre un traitement continu de 80 mg de tobramycine deux fois par jour ou de 300 mg de tobramycine deux fois par jour par cycle de 28 jours de traitement suivis de 28 jours sans traitement. Au bout de trois mois, les patients sont passés à l’autre schéma thérapeutique.
RÉSULTATS :: Au total, 32 patients, d’un âge moyen (±ÉT) de 18,5±8,6 ans, ont participé à l’étude. Par rapport à la période de traitement à la colistine, le volume expiratoire maximal par seconde (VEMS) a diminué de −2,1±13,8 % dans le groupe prenant 80 mg de tobramycine et a augmenté de +2,3±13,0 % dans celui en prenant 300 mg. On a observé des changements similaires de la capacité vitale forcée (−2,5±12,9 % dans le groupe prenant 80 mg de tobramycine par rapport à +2,5±9,6 % dans celui en prenant 300 mg). Les auteurs ont constaté une vaste variabilité des réponses, mais les différences n’étaient pas statistiquement significatives. Pour ce qui est des préférences personnelles, la majorité des patients ont préféré le cycle à fortes doses par rapport à l’inhalation continue à faible dose, mais cette observation n’était pas reliée à des données objectives d’efficacité.
CONCLUSIONS :: Le présent essai ne fournit pas de données probantes convaincantes sur l’efficacité supérieure de l’un ou l’autre des schémas thérapeutiques de tobramycine en aérosol chez les patients atteints de FK.
Figures

References
-
- Gappa M, Steinkamp G, Tümmler B, von der Hardt H. Long-term tobramycin aerosol therapy of chronic Pseudomonas aeruginosa infection in patients with cystic fibrosis. Scand J Gastroenterol Suppl. 1988;143:74–6. - PubMed
-
- MacLusky IB, Gold R, Corey M, Levison H. Long-term effects of inhaled tobramycin in patients with cystic fibrosis colonized with Pseudomonas aeruginosa. Pediatr Pulmonol. 1989;7:42–8. - PubMed
-
- Ryan G, Mukhopadhyay S, Singh M. Nebulised anti-pseudomonal antibiotics for cystic fibrosis. Cochrane Database Syst Rev. 2000. p. CD001021. - PubMed
-
- Ramsey BW, Pepe MS, Quan JM, et al. Intermittent administration of inhaled tobramycin in patients with cystic fibrosis. Cystic Fibrosis Inhaled Tobramycin Study Group. N Engl J Med. 1999;340:23–30. - PubMed
-
- Polgar G, Promadhat V. Pulmonary Function Testing in Children: Techniques and Standards. Philadelphia: WB Saunders; 1971.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

