Inhibition of ref-1 stimulates the production of reactive oxygen species and induces differentiation in adult cardiac stem cells
- PMID: 18717627
- PMCID: PMC2933566
- DOI: 10.1089/ars.2008.2195
Inhibition of ref-1 stimulates the production of reactive oxygen species and induces differentiation in adult cardiac stem cells
Abstract
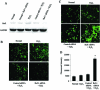
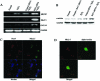
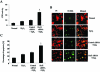
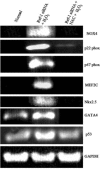
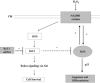
Redox effector protein-1 (Ref-1) plays an essential role in DNA repair and redox regulation of several transcription factors. In the present study, we examined the role of Ref-1 in maintaining the redox status and survivability of adult cardiac stem cells challenged with a subtoxic level of H2O2 under inhibition of Ref-1 by RNA interference. Treatment of cardiac stem cells with a low concentration of H2O2 induced Ref-1-mediated survival signaling through phosphorylation of Akt. However, Ref-1 inhibition followed by H2O2 treatment extensively induced the level of intracellular reactive oxygen species (ROS) through activation of the components of NADPH oxidase, like p22( phox ), p47( phox ), and Nox4. Cardiac differentiation markers (Nkx2.5, MEF2C, and GATA4), and cell death by apoptosis were significantly elevated in Ref-1 siRNA followed by H2O2-treated stem cells. Further, inhibition of Ref-1 increased the level of p53 but decreased the phosphorylation of Akt, a molecule involved in survival signaling. Treatment with ROS scavenger N-acetyl-L-cysteine attenuated Ref-1 siRNA-mediated activation of NADPH oxidase and cardiac differentiation. Taken together, these results indicate that Ref-1 plays an important role in maintaining the redox status of cardiac stem cells and protects them from oxidative injury-mediated cell death and differentiation.
Figures


References
-
- Allen RG. Venkatraj VS. Oxidants and antioxidants in development and differentiation. J Nutr. 1992;122:631–635. - PubMed
-
- Almog N. Rotter V. Involvement of p53 in cell differentiation and development. Biochim Biophys Acta. 1997;1333:F1–F27. - PubMed
-
- Angkeow P. Deshpande SS. Qi B. Liu YX. Park YC. Jeon BH. Ozaki M. Irani K. Redox factor-1: an extra-nuclear role in the regulation of endothelial oxidative stress and apoptosis. Cell Death Differ. 2002;9:717–725. - PubMed
-
- Beckman BS. Balin AK. Allen RG. Superoxide dismutase induces differentiation of Friend erythroleukemia cells. J Cell Physiol. 1989;139:370–376. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

