Optimization of capsid-incorporated antigens for a novel adenovirus vaccine approach
- PMID: 18718011
- PMCID: PMC2535600
- DOI: 10.1186/1743-422X-5-98
Optimization of capsid-incorporated antigens for a novel adenovirus vaccine approach
Abstract
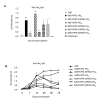
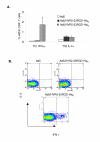
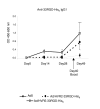
Despite the many potential advantages of Ad vectors for vaccine application, the full utility of current Ad vaccines may be limited by the host anti-vector immune response. Direct incorporation of antigens into the adenovirus capsid offers a new and exciting approach for vaccination strategies; this strategy exploits the inherent antigenicity of the Ad vector. Critical to exploiting Ad in this new context is the placement of antigenic epitopes within the major Ad capsid protein, hexon. In our current study we illustrate that we have the capability to place a range of antigenic epitopes within Ad5 capsid protein hexon hypervariable regions (HVRs) 2 or 5, thus producing viable Ad virions. Our data define the maximal incorporation size at HVR2 or HVR5 as it relates to identical antigenic epitopes. In addition, this data suggests that Ad5 HVR5 is more permissive to a range of insertions. Most importantly, repeated administration of our hexon-modified viruses resulted in a secondary anti-antigen response, whereas minimal secondary effect was present after administration of Ad5 control. Our study describes antigen placement and optimization within the context of the capsid incorporation approach of Ad vaccine employment, thereby broadening this new methodology.
Figures



References
-
- Dmitriev I, Krasnykh V, Miller CR, Wang M, Kashentseva E, Mikheeva G, Belousova N, Curiel DT. An adenovirus vector with genetically modified fibers demonstrates expanded tropism via utilization of a coxsackievirus and adenovirus receptor-independent cell entry mechanism. J Virol. 1998;72:9706–9713. - PMC - PubMed
-
- Barnett BG, Crews CJ, Douglas JT. Targeted adenoviral vectors. Biochim Biophys Acta. 2002;1575:1–14. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

