Catabolite repression control of flagellum production by Serratia marcescens
- PMID: 18718529
- PMCID: PMC2606049
- DOI: 10.1016/j.resmic.2008.07.003
Catabolite repression control of flagellum production by Serratia marcescens
Abstract
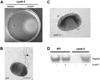
Serratia marcescens is an emerging opportunistic pathogen with a remarkably broad host range. The cAMP-regulated catabolite repression system of S. marcescens has recently been identified and demonstrated to regulate biofilm formation through the production of surface adhesions. Here we report that mutations in components of the catabolite repression system (cyaA and crp) eliminate flagellum production and swimming motility. Exogenous cAMP was able to restore flagellum production to adenylate cyclase mutants, as determined by transmission electron microscopy and PAGE analysis. A transposon-generated suppressor mutation of the crp motility defect mapped to upstream of the flhDC operon. This suppressor mutation resulted in an upregulation of flhD expression and flagellum production, indicating that flhDC expression is sufficient to restore flagellum production to crp mutants. Lastly, and contrary to a previous report, we found that flhD expression is controlled by the catabolite repression system using quantitative RT-PCR. Together, these data indicate that flagellum production is regulated by the cAMP-dependent catabolite repression system. Given the role of flagella in bacterial pathogenicity, the regulatory pathway described here may assist us in better understanding the putative role of motility in dissemination and virulence of this opportunistic pathogen.
Figures





References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

