Dynamics of neutrophil migration in lymph nodes during infection
- PMID: 18718768
- PMCID: PMC2569002
- DOI: 10.1016/j.immuni.2008.07.012
Dynamics of neutrophil migration in lymph nodes during infection
Erratum in
- Immunity. 2008 Oct;29(4):661
Abstract
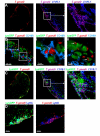
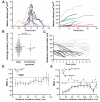
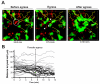
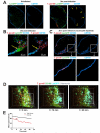
Although the signals that control neutrophil migration from the blood to sites of infection have been well characterized, little is known about their migration patterns within lymph nodes or the strategies that neutrophils use to find their local sites of action. To address these questions, we used two-photon scanning-laser microscopy to examine neutrophil migration in intact lymph nodes during infection with an intracellular parasite, Toxoplasma gondii. We found that neutrophils formed both small, transient and large, persistent swarms via a coordinated migration pattern. We provided evidence that cooperative action of neutrophils and parasite egress from host cells could trigger swarm formation. Neutrophil swarm formation coincided in space and time with the removal of macrophages that line the subcapsular sinus of the lymph node. Our data provide insights into the cellular mechanisms underlying neutrophil swarming and suggest new roles for neutrophils in shaping immune responses.
Figures

References
-
- Abadie V, Badell E, Douillard P, Ensergueix D, Leenen PJ, Tanguy M, Fiette L, Saeland S, Gicquel B, Winter N. Neutrophils rapidly migrate via lymphatics after Mycobacterium bovis BCG intradermal vaccination and shuttle live bacilli to the draining lymph nodes. Blood. 2005;106:1843–1850. - PubMed
-
- Adachi O, Kawai T, Takeda K, Matsumoto M, Tsutsui H, Sakagami M, Nakanishi K, Akira S. Targeted disruption of the MyD88 gene results in loss of IL-1- and IL-18-mediated function. Immunity. 1998;9:143–150. - PubMed
-
- Appelberg R. Neutrophils and intracellular pathogens: beyond phagocytosis and killing. Trends in microbiology. 2007;15:87–92. - PubMed
-
- Baggiolini M. Chemokines and leukocyte traffic. Nature. 1998;392:565–568. - PubMed
-
- Beauvillain C, Delneste Y, Scotet M, Peres A, Gascan H, Guermonprez P, Barnaba V, Jeannin P. Neutrophils efficiently cross-prime naive T cells in vivo. Blood. 2007;110:2965–2973. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

