Depletion of central BDNF in mice impedes terminal differentiation of new granule neurons in the adult hippocampus
- PMID: 18718867
- PMCID: PMC2652348
- DOI: 10.1016/j.mcn.2008.07.017
Depletion of central BDNF in mice impedes terminal differentiation of new granule neurons in the adult hippocampus
Abstract
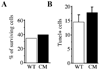
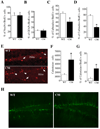
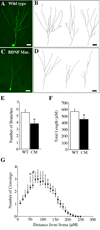
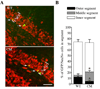
Granule neurons generated in the adult mammalian hippocampus synaptically integrate to facilitate cognitive function and antidepressant efficacy. Here, we investigated the role of BDNF in facilitating their maturation in vivo. We found that depletion of central BDNF in mice elicited an increase in hippocampal cell proliferation without affecting cell survival or fate specification. However, new mutant neurons failed to fully mature as indicated by their lack of calbindin, reduced dendritic differentiation and an accumulation of calretinin(+) immature neurons in the BDNF mutant dentate gyrus. Furthermore, the facilitating effects of GABA(A) receptor stimulation on neurogenesis were absent in the mutants, suggesting that defects might be due to alterations in GABA signaling. Transcriptional analysis of the mutant hippocampal neurogenic region revealed increases in markers for immature neurons and decreases in neuronal differentiation facilitators. These findings demonstrate that BDNF is required for the terminal differentiation of new neurons in the adult hippocampus.
Figures





References
-
- Ambrogini P, Lattanzi D, Ciuffoli S, Agostini D, Bertini L, Stocchi V, Santi S, Cuppini R. Morpho-functional characterization of neuronal cells at different stages of maturation in granule cell layer of adult rat dentate gyrus. Brain Res. 2004;1017:21–31. - PubMed
-
- Biebl M, Cooper CM, Winkler J, Kuhn HG. Analysis of neurogenesis and programmed cell death reveals a self-renewing capacity in the adult rat brain. Neurosci Lett. 2000;291:17–20. - PubMed
-
- Borghesani PR, Peyrin JM, Klein R, Rubin J, Carter AR, Schwartz PM, Luster A, Corfas G, Segal RA. BDNF stimulates migration of cerebellar granule cells. Development. 2002;129:1435–1442. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

