Follistatin-like 1, a secreted muscle protein, promotes endothelial cell function and revascularization in ischemic tissue through a nitric-oxide synthase-dependent mechanism
- PMID: 18718903
- PMCID: PMC2583310
- DOI: 10.1074/jbc.M803440200
Follistatin-like 1, a secreted muscle protein, promotes endothelial cell function and revascularization in ischemic tissue through a nitric-oxide synthase-dependent mechanism
Abstract
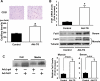
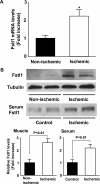
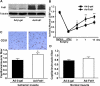
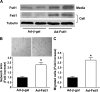
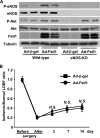
Myogenic Akt signaling coordinates blood vessel recruitment with normal tissue growth. Here, we investigated the role of Follistatin-like 1 (Fstl1) in the regulation of endothelial cell function and blood vessel growth in muscle. Transgenic Akt1 overexpression in skeletal muscle led to myofiber growth that was coupled to an increase in muscle capillary density. Myogenic Akt signaling or ischemic hind limb surgery led to the induction of Fstl1 in muscle and increased circulating levels of Fstl1. Intramuscular administration of an adenoviral vector expressing Fstl1 (Ad-Fstl1) accelerated flow recovery and increased capillary density in the ischemic hind limbs of wild-type mice, and this was associated with an increase in endothelial nitric oxide synthase (eNOS) phosphorylation at residue Ser-1179. In cultured endothelial cells, Ad-Fstl1 stimulated migration and differentiation into network structures and inhibited apoptosis under conditions of serum deprivation. These cell responses were associated with the activating phosphorylation of Akt and eNOS. Conversely, transduction with dominant-negative Akt or LY294002 blocked Fstl1-stimulated eNOS phosphorylation and inhibited Fstl1-stimulated cellular responses. Treatment with the eNOS inhibitor N(G)-nitro-L-arginine methyl ester also reduced endothelial cell migration and differentiation induced by Ad-Fstl1. The stimulatory effect of Ad-Fstl1 on ischemic limb reperfusion was abolished in mice lacking eNOS. These data indicate that Fstl1 is a secreted muscle protein or myokine that can function to promote endothelial cell function and stimulates revascularization in response to ischemic insult through its ability to activate Akt-eNOS signaling.
Figures



Similar articles
-
Fat-derived factor omentin stimulates endothelial cell function and ischemia-induced revascularization via endothelial nitric oxide synthase-dependent mechanism.J Biol Chem. 2012 Jan 2;287(1):408-417. doi: 10.1074/jbc.M111.261818. Epub 2011 Nov 11. J Biol Chem. 2012. PMID: 22081609 Free PMC article.
-
Aerobic exercise training-induced follistatin-like 1 secretion in the skeletal muscle is related to arterial stiffness via arterial NO production in obese rats.Physiol Rep. 2022 May;10(10):e15300. doi: 10.14814/phy2.15300. Physiol Rep. 2022. PMID: 35585770 Free PMC article.
-
Diallyl Trisulfide Augments Ischemia-Induced Angiogenesis via an Endothelial Nitric Oxide Synthase-Dependent Mechanism.Circ J. 2017 May 25;81(6):870-878. doi: 10.1253/circj.CJ-16-1097. Epub 2017 Feb 17. Circ J. 2017. PMID: 28216514
-
The role of endothelial insulin signaling in the regulation of glucose metabolism.Rev Endocr Metab Disord. 2013 Jun;14(2):207-16. doi: 10.1007/s11154-013-9242-z. Rev Endocr Metab Disord. 2013. PMID: 23589150 Review.
-
Follistatin-like 1 in development and human diseases.Cell Mol Life Sci. 2018 Jul;75(13):2339-2354. doi: 10.1007/s00018-018-2805-0. Epub 2018 Mar 29. Cell Mol Life Sci. 2018. PMID: 29594389 Free PMC article. Review.
Cited by
-
FSTL1 as a Potential Mediator of Exercise-Induced Cardioprotection in Post-Myocardial Infarction Rats.Sci Rep. 2016 Aug 26;6:32424. doi: 10.1038/srep32424. Sci Rep. 2016. PMID: 27561749 Free PMC article.
-
SILAC-based quantitative proteomic analysis of gastric cancer secretome.Proteomics Clin Appl. 2013 Jun;7(5-6):355-66. doi: 10.1002/prca.201200069. Epub 2013 May 21. Proteomics Clin Appl. 2013. PMID: 23161554 Free PMC article.
-
Follistatin-like 1 and its paralogs in heart development and cardiovascular disease.Heart Fail Rev. 2022 Nov;27(6):2251-2265. doi: 10.1007/s10741-022-10262-6. Epub 2022 Jul 22. Heart Fail Rev. 2022. PMID: 35867287 Free PMC article. Review.
-
Neuron-derived neurotrophic factor functions as a novel modulator that enhances endothelial cell function and revascularization processes.J Biol Chem. 2014 May 16;289(20):14132-44. doi: 10.1074/jbc.M114.555789. Epub 2014 Apr 6. J Biol Chem. 2014. PMID: 24706764 Free PMC article.
-
Muscle-derived follistatin-like 1 functions to reduce neointimal formation after vascular injury.Cardiovasc Res. 2014 Jul 1;103(1):111-20. doi: 10.1093/cvr/cvu105. Epub 2014 Apr 17. Cardiovasc Res. 2014. PMID: 24743592 Free PMC article.
References
-
- Degens, H., Veerkamp, J. H., van Moerkerk, H. T. B., Turek, Z., Hoofd, L. J. C., and Binkhorst, R. A. (1993) Int. J. Biochem. 25 1141-1148 - PubMed
-
- Ingjer, F. (1979) Eur. J. Appl. Physiol. Occup. Physiol. 40 197-209 - PubMed
-
- Kano, Y., Shimegi, S., Masuda, K., Ohmori, H., and Katsuta, S. (1997) Eur. J. Appl. Physiol. Occup. Physiol. 75 97-101 - PubMed
-
- Shiojima, I., and Walsh, K. (2006) Genes Dev. 20 3347-3365 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

