Targeted induction of lung endothelial cell apoptosis causes emphysema-like changes in the mouse
- PMID: 18718906
- PMCID: PMC2570855
- DOI: 10.1074/jbc.M804595200
Targeted induction of lung endothelial cell apoptosis causes emphysema-like changes in the mouse
Abstract
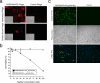
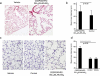
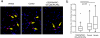
Pulmonary gas exchange relies on a rich capillary network, which, together with alveolar epithelial type I and II cells, form alveolar septa, the functional units in the lung. Alveolar capillary endothelial cells are critical in maintaining alveolar structure, because disruption of endothelial cell integrity underlies several lung diseases. Here we show that targeted ablation of lung capillary endothelial cells recapitulates the cellular events involved in cigarette smoke-induced emphysema, one of the most prevalent nonneoplastic lung diseases. Based on phage library screening on an immortalized lung endothelial cell line, we identified a lung endothelial cell-binding peptide, which preferentially homes to lung blood vessels. This peptide fused to a proapoptotic motif specifically induced programmed cell death of lung endothelial cells in vitro as well as targeted apoptosis of the lung microcirculation in vivo. As early as 4 days following peptide administration, mice developed air space enlargement associated with enhanced oxidative stress, influx of macrophages, and up-regulation of ceramide. Given that these are all critical elements of the corresponding human emphysema caused by cigarette smoke, these data provide evidence for a central role for the alveolar endothelial cells in the maintenance of lung structure and of endothelial cell apoptosis in the pathogenesis of emphysema-like changes. Thus, our data enable the generation of a convenient mouse model of human emphysema. Finally, combinatorial screenings on immortalized cells followed by in vivo targeting establishes an experimental framework for discovery and validation of additional ligand-directed pharmacodelivery systems.
Figures




References
-
- Sirianni, F. E., Chu, F. S., and Walker, D. C. (2003) Am. J. Respir. Crit. Care Med. 168 1532-1537 - PubMed
-
- Snider, G. L., Kleinerman, J. L., Thurlbeck, W. M., and Bengali, Z. H. (1985) Am. Rev. Respir. Dis. 132 182-185 - PubMed
-
- Shapiro, S. D. (1995) Proc. Assoc. Am. Physicians 107 346-352 - PubMed
-
- Liebow, A. A. (1959) Am. Rev. Respir. Dis. 80 67-93 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

