Induction of pyruvate dehydrogenase kinase-3 by hypoxia-inducible factor-1 promotes metabolic switch and drug resistance
- PMID: 18718909
- PMCID: PMC2661383
- DOI: 10.1074/jbc.M803508200
Induction of pyruvate dehydrogenase kinase-3 by hypoxia-inducible factor-1 promotes metabolic switch and drug resistance
Abstract
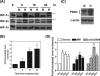
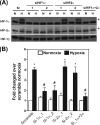
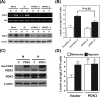
The switch of cellular metabolism from mitochondrial respiration to glycolysis is the hallmark of cancer cells and associated with tumor malignancy. However, the mechanism of this metabolic switch remains largely unknown. Herein, we reported that hypoxia-inducible factor-1 (HIF-1) induced pyruvate dehydrogenase kinase-3 (PDK3) expression leading to inhibition of mitochondrial respiration. Promoter activity assay, small interference RNA knockdown assay, and chromatin immunoprecipitation assay demonstrated that hypoxia-induced PDK3 gene activity was regulated by HIF-1 at the transcriptional level. Forced expression of PDK3 in cancer cells resulted in increased lactic acid accumulation and drugs resistance, whereas knocking down PDK3 inhibited hypoxia-induced cytoplasmic glycolysis and cell survival. These data demonstrated that increased PDK3 expression due to elevated HIF-1alpha in cancer cells may play critical roles in metabolic switch during cancer progression and chemoresistance in cancer therapy.
Figures



Similar articles
-
Overexpression of pyruvate dehydrogenase kinase 3 increases drug resistance and early recurrence in colon cancer.Am J Pathol. 2011 Sep;179(3):1405-14. doi: 10.1016/j.ajpath.2011.05.050. Epub 2011 Jul 18. Am J Pathol. 2011. PMID: 21763680 Free PMC article.
-
Hypoxia-inducible factor 1 and dysregulated c-Myc cooperatively induce vascular endothelial growth factor and metabolic switches hexokinase 2 and pyruvate dehydrogenase kinase 1.Mol Cell Biol. 2007 Nov;27(21):7381-93. doi: 10.1128/MCB.00440-07. Epub 2007 Sep 4. Mol Cell Biol. 2007. PMID: 17785433 Free PMC article.
-
Metabolic enzyme PDK3 forms a positive feedback loop with transcription factor HSF1 to drive chemoresistance.Theranostics. 2019 May 9;9(10):2999-3013. doi: 10.7150/thno.31301. eCollection 2019. Theranostics. 2019. PMID: 31244938 Free PMC article.
-
Hypoxia-inducible factor 1: regulator of mitochondrial metabolism and mediator of ischemic preconditioning.Biochim Biophys Acta. 2011 Jul;1813(7):1263-8. doi: 10.1016/j.bbamcr.2010.08.006. Epub 2010 Aug 21. Biochim Biophys Acta. 2011. PMID: 20732359 Free PMC article. Review.
-
Regulation of cancer cell metabolism by hypoxia-inducible factor 1.Semin Cancer Biol. 2009 Feb;19(1):12-6. doi: 10.1016/j.semcancer.2008.11.009. Epub 2008 Dec 9. Semin Cancer Biol. 2009. PMID: 19114105 Review.
Cited by
-
A novel PDHK inhibitor restored cognitive dysfunction and limited neurodegeneration without affecting amyloid pathology in 5xFAD mouse, a model of Alzheimer's disease.Alzheimers Res Ther. 2024 Sep 5;16(1):197. doi: 10.1186/s13195-024-01552-2. Alzheimers Res Ther. 2024. PMID: 39238036 Free PMC article.
-
Blocking Aerobic Glycolysis by Targeting Pyruvate Dehydrogenase Kinase in Combination with EGFR TKI and Ionizing Radiation Increases Therapeutic Effect in Non-Small Cell Lung Cancer Cells.Cancers (Basel). 2021 Feb 24;13(5):941. doi: 10.3390/cancers13050941. Cancers (Basel). 2021. PMID: 33668151 Free PMC article.
-
ROS homeostasis and metabolism: a critical liaison for cancer therapy.Exp Mol Med. 2016 Nov 4;48(11):e269. doi: 10.1038/emm.2016.119. Exp Mol Med. 2016. PMID: 27811934 Free PMC article. Review.
-
Reducing Oxidative Stress and Inflammation by Pyruvate Dehydrogenase Kinase 4 Inhibition Is Important in Prevention of Renal Ischemia-Reperfusion Injury in Diabetic Mice.Diabetes Metab J. 2024 May;48(3):405-417. doi: 10.4093/dmj.2023.0196. Epub 2024 Feb 1. Diabetes Metab J. 2024. PMID: 38311057 Free PMC article.
-
Energy metabolic shift contributes to the phenotype modulation of maturation stage ameloblasts.Front Physiol. 2022 Nov 29;13:1062042. doi: 10.3389/fphys.2022.1062042. eCollection 2022. Front Physiol. 2022. PMID: 36523561 Free PMC article.
References
-
- Warburg, O., Wind, F., and Neglers, E. (1930) The Metabolism of Tumors Constable and Co., London, pp. 254–270
-
- Greijer, A. E., van der Groep, P., Kemming, D., Shvarts, A., Semenza, G. L., Meijer, G. A., van de Wiel, M. A., Belien, J. A., van Diest, P. J., and van der Wall, E. (2005) J. Pathol. 206 291–304 - PubMed
-
- Patel, M. S., and Korotchkina, L. G. (2001) Exp. Mol. Med. 33 191–197 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

