Characterization of thyrotropin receptor antibody-induced signaling cascades
- PMID: 18719020
- PMCID: PMC2630889
- DOI: 10.1210/en.2008-0878
Characterization of thyrotropin receptor antibody-induced signaling cascades
Abstract
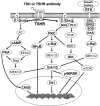
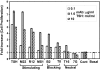
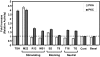
The TSH receptor (TSHR) is constitutively active and is further enhanced by TSH ligand binding or by stimulating TSHR antibodies (TSHR-Abs) as seen in Graves' disease. TSH is known to activate the thyroid epithelial cell via both Galphas-cAMP/protein kinase A/ERK and Galphaq-Akt/protein kinase C coupled signaling networks. The recent development of monoclonal antibodies to the TSHR has enabled us to investigate the hypothesis that different TSHR-Abs may have unique signaling imprints that differ from TSH ligand itself. We have, therefore, performed sequential studies, using rat thyrocytes (FRTL-5, passages 5-20) as targets, to examine the signaling pathways activated by a series of monoclonal TSHR-Abs in comparison with TSH itself. Activation of key signaling molecules was estimated by specific immunoblots and/or enzyme immunoassays. Continuing constitutive TSHR activity in thyroid cells, deprived of TSH and serum for 48 h, was demonstrated by pathway-specific chemical inhibition. Under our experimental conditions, TSH ligand and TSHR-stimulating antibodies activated both Galphas and Galphaq effectors. Importantly, some TSHR-blocking and TSHR-neutral antibodies were also able to generate signals, influencing primarily the Galphaq effectors and induced cell proliferation. Most strikingly, antibodies that used the Galphaq cascades used c-Raf-ERK-p90RSK as a unique signaling cascade not activated by TSH. Our study demonstrated that individual TSHR-Abs had unique molecular signatures which resulted in sequential preferences. Because downstream thyroid cell signaling by the TSHR is both ligand dependent and independent, this may explain why TSHR-Abs are able to have variable influences on thyroid cell biology.
Figures


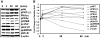


References
-
- Kimura T, Van KA, Golstein J, Fusco A, Dumont JE, Roger PP 2001 Regulation of thyroid cell proliferation by TSH and other factors: a critical evaluation of in vitro models. Endocr Rev 22:631–656 - PubMed
-
- Medina DL, Santisteban P 2000 Thyrotropin-dependent proliferation of in vitro rat thyroid cell systems. Eur J Endocrinol 143:161–178 - PubMed
-
- Bachrach LK, Eggo MC, Mak WW, Burrow GN 1985 Phorbol esters stimulate growth and inhibit differentiation in cultured thyroid cells. Endocrinology 116:1603–1609 - PubMed
-
- Roger PP, Reuse S, Servais P, Van HB, Dumont JE 1986 Stimulation of cell proliferation and inhibition of differentiation expression by tumor-promoting phorbol esters in dog thyroid cells in primary culture. Cancer Res 46:898–906 - PubMed
-
- Morrison DK, Cutler RE 1997 The complexity of Raf-1 regulation. Curr Opin Cell Biol 9:174–179 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

