In vivo human choroidal thickness measurements: evidence for diurnal fluctuations
- PMID: 18719079
- PMCID: PMC4112498
- DOI: 10.1167/iovs.08-1779
In vivo human choroidal thickness measurements: evidence for diurnal fluctuations
Abstract
Purpose: The authors applied partial coherence interferometry (PCI) to estimate the thickness of the human choroid in vivo and to learn whether it fluctuates during the day.
Methods: By applying signal processing techniques to existing PCI tracings of human ocular axial length measurements, a signal modeling algorithm was developed and validated to determine the position and variability of a postretinal peak that, by analogy to animal studies, likely corresponds to the choroidal/scleral interface. The algorithm then was applied to diurnal axial eye length datasets.
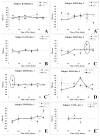
Results: The postretinal peak was identified in 28% of subjects in the development and validation datasets, with mean subfoveal choroidal thicknesses of 307 and 293 microm, respectively. Twenty-eight of 40 diurnal PCI datasets had at least two time points with identifiable postretinal peaks, yielding a mean choroidal thickness of 426 microm and a mean high-low difference in choroidal thickness of 59.5 +/- 24.2 microm (range, 25.9-103 microm). The diurnal choroidal thickness fluctuation was larger than twice the SE of measurement (24.5 microm) in 16 of these 28 datasets. Axial length and choroidal thickness tended to fluctuate in antiphase.
Conclusions: Signal processing techniques provide choroidal thickness estimates in many, but not all, PCI datasets of axial eye measurements. Based on eyes with identifiable postretinal peaks at more than one time in a day, choroidal thickness varied over the day. Because of the established role of the choroid in retinal function and its possible role in regulating eye growth, further development and refinement of clinical methods to measure its thickness are warranted.
Figures
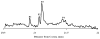
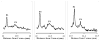


Similar articles
-
Ocular axial length and choroidal thickness in newly hatched chicks and one-year-old chickens fluctuate in a diurnal pattern that is influenced by visual experience and intraocular pressure changes.Exp Eye Res. 1998 Feb;66(2):195-205. doi: 10.1006/exer.1997.0421. Exp Eye Res. 1998. PMID: 9533845
-
Daily axial length and choroidal thickness variations in young adults: Associations with light exposure and longitudinal axial length and choroid changes.Exp Eye Res. 2019 Dec;189:107850. doi: 10.1016/j.exer.2019.107850. Epub 2019 Oct 19. Exp Eye Res. 2019. PMID: 31639338
-
Diurnal axial length fluctuations in human eyes.Invest Ophthalmol Vis Sci. 2004 Jan;45(1):63-70. doi: 10.1167/iovs.03-0294. Invest Ophthalmol Vis Sci. 2004. PMID: 14691155
-
Ocular diurnal rhythms and eye growth regulation: where we are 50 years after Lauber.Exp Eye Res. 2013 Sep;114:25-34. doi: 10.1016/j.exer.2012.12.013. Epub 2013 Jan 5. Exp Eye Res. 2013. PMID: 23298452 Free PMC article. Review.
-
[A new approach for studying the retinal and choroidal circulation].Nippon Ganka Gakkai Zasshi. 2004 Dec;108(12):836-61; discussion 862. Nippon Ganka Gakkai Zasshi. 2004. PMID: 15656089 Review. Japanese.
Cited by
-
Change in subfoveal choroidal thickness in central serous chorioretinopathy following spontaneous resolution and low-fluence photodynamic therapy.Eye (Lond). 2013 Mar;27(3):387-91. doi: 10.1038/eye.2012.273. Epub 2013 Jan 4. Eye (Lond). 2013. PMID: 23288136 Free PMC article.
-
Effects of Narrowband Light on Choroidal Thickness and the Pupil.Invest Ophthalmol Vis Sci. 2020 Aug 3;61(10):40. doi: 10.1167/iovs.61.10.40. Invest Ophthalmol Vis Sci. 2020. PMID: 32832970 Free PMC article.
-
[Are there diurnal variations in choroidal thickness?].Ophthalmologe. 2015 Aug;112(8):665-9. doi: 10.1007/s00347-014-3177-y. Ophthalmologe. 2015. PMID: 25566738 German.
-
Myopic defocus in the evening is more effective at inhibiting eye growth than defocus in the morning: Effects on rhythms in axial length and choroid thickness in chicks.Exp Eye Res. 2017 Jan;154:104-115. doi: 10.1016/j.exer.2016.11.012. Epub 2016 Nov 11. Exp Eye Res. 2017. PMID: 27845062 Free PMC article.
-
Effect of pupil dilation on macular choroidal thickness measured with spectral domain optical coherence tomography in normal and glaucomatous eyes.Int Ophthalmol. 2013 Aug;33(4):335-41. doi: 10.1007/s10792-012-9689-z. Epub 2013 Jan 1. Int Ophthalmol. 2013. PMID: 23277206
References
-
- Wallman J, Wildsoet C, Xu A, et al. Moving the retina: choroidal modulation of refractive state. Vision Res. 1995;35:37–50. - PubMed
-
- Hung L, Wallman J, Smith ER. Vision-dependent changes in the choroidal thickness of macaque monkeys. Invest Ophthalmol Vis Sci. 2000;41:1259–1269. - PubMed
-
- Troilo D, Nickla D, Wildsoet C. Choroidal thickness changes during altered eye growth and refractive state in a primate. Invest Ophthalmol Vis Sci. 2000;41:1249–1258. - PubMed
-
- Nickla D. The phase relationships between the diurnal rhythms in axial length and choroidal thickness and the association with ocular growth rate in chicks. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2006;192:399–407. - PubMed
-
- Nickla D, Wildsoet C, Wallman J. The circadian rhythm in intraocular pressure and its relation to diurnal ocular growth changes in chicks. Exp Eye Res. 1998;66:183–193. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

