Methionine oxidation impairs reverse cholesterol transport by apolipoprotein A-I
- PMID: 18719109
- PMCID: PMC2527893
- DOI: 10.1073/pnas.0802025105
Methionine oxidation impairs reverse cholesterol transport by apolipoprotein A-I
Abstract
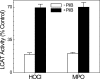
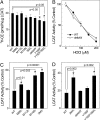
HDL protects against vascular disease by accepting free cholesterol from macrophage foam cells in the artery wall. This pathway is critically dependent on lecithin:cholesterol acyltransferase (LCAT), which rapidly converts cholesterol to cholesteryl ester. The physiological activator of LCAT is apolipoprotein A-I (apoA-I), the major HDL protein. However, cholesterol removal is compromised if apoA-I is exposed to reactive intermediates. In humans with established cardiovascular disease, myeloperoxidase (MPO) oxidizes HDL, and oxidation by MPO impairs apoA-I's ability to activate LCAT in vitro. Because a single methionine residue in apoA-I, Met-148, resides near the center of the protein's LCAT activation domain, we determined whether its oxidation by MPO could account for the loss of LCAT activity. Mass spectrometric analysis demonstrated that oxidation of Met-148 to methionine sulfoxide associated quantitatively with loss of LCAT activity in both discoidal HDL and HDL(3), the enzyme's physiological substrates. Reversing oxidation with methionine sulfoxide reductase restored HDL's ability to activate LCAT. Discoidal HDL prepared with apoA-I containing a Met-148-->Leu mutation was significantly resistant to inactivation by MPO. Based on structural data in the literature, we propose that oxidation of Met-148 disrupts apoA-I's central loop, which overlaps the LCAT activation domain. These observations implicate oxidation of a single Met in apoA-I in impaired LCAT activation, a critical early step in reverse cholesterol transport.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Gordon DJ, Rifkind BM. High-density lipoprotein-the clinical implications of recent studies. N Engl J Med. 1989;321:1311–1316. - PubMed
-
- Glomset JA. The plasma lecithin:Cholesterol acyltransferase reaction. J Lipid Res. 1968;9:155–167. - PubMed
-
- Oram JF, Heinecke JW. ATP-binding cassette transporter A1: A cell cholesterol exporter that protects against cardiovascular disease. Physiol Rev. 2005;85:1343–1372. - PubMed
-
- Jonas A. Lecithin-cholesterol acyltransferase in the metabolism of high-density lipoproteins. Biochim Biophys Acta. 1991;1084:205–220. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

