Stress resistance and signal fidelity independent of nuclear MAPK function
- PMID: 18719124
- PMCID: PMC2518827
- DOI: 10.1073/pnas.0805797105
Stress resistance and signal fidelity independent of nuclear MAPK function
Abstract
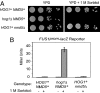
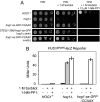
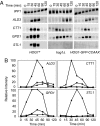
Elevated external solute stimulates a conserved MAPK cascade that elicits responses that maintain osmotic balance. The yeast high-osmolarity glycerol (HOG) pathway activates Hog1 MAPK (mammalian ortholog p38alpha/SAPKalpha), which enters the nucleus and induces expression of >50 genes, implying that transcriptional up-regulation is necessary to cope with hyperosmotic stress. Contrary to this expectation, we show here that cells lacking the karyopherin required for Hog1 nuclear import or in which Hog1 is anchored at the plasma membrane (or both) can withstand long-term hyperosmotic challenge by ionic and nonionic solutes without exhibiting the normal change in transcriptional program (comparable with hog1Delta cells), as judged by mRNA hybridization and microarray analysis. For such cells to survive hyperosmotic stress, systematic genetic analysis ruled out the need for any Hog1-dependent transcription factor, the Hog1-activated MAPKAP kinases, or ion, glycerol, and water channels. By contrast, enzymes needed for glycerol production were essential for viability. Thus, control of intracellular glycerol formation by Hog1 is critical for maintenance of osmotic balance but not transcriptional induction of any gene.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

