Human amniotic fluid stem cells can integrate and differentiate into epithelial lung lineages
- PMID: 18719226
- PMCID: PMC3174105
- DOI: 10.1634/stemcells.2008-0090
Human amniotic fluid stem cells can integrate and differentiate into epithelial lung lineages
Abstract
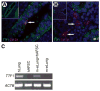
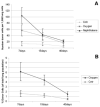
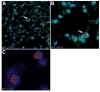
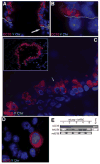
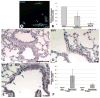
A new source of stem cells has recently been isolated from amniotic fluid; these amniotic fluid stem cells have significant potential for regenerative medicine. These cells are multipotent, showing the ability to differentiate into cell types from each embryonic germ layer. We investigated the ability of human amniotic fluid stem cells (hAFSC) to integrate into murine lung and to differentiate into pulmonary lineages after injury. Using microinjection into cultured mouse embryonic lungs, hAFSC can integrate into the epithelium and express the early human differentiation marker thyroid transcription factor 1 (TTF1). In adult nude mice, following hyperoxia injury, tail vein-injected hAFSC localized in the distal lung and expressed both TTF1 and the type II pneumocyte marker surfactant protein C. Specific damage of Clara cells through naphthalene injury produced integration and differentiation of hAFSC at the bronchioalveolar and bronchial positions with expression of the specific Clara cell 10-kDa protein. These results illustrate the plasticity of hAFSC to respond in different ways to different types of lung damage by expressing specific alveolar versus bronchiolar epithelial cell lineage markers, depending on the type of injury to recipient lung. Disclosure of potential conflicts of interest is found at the end of this article.
Conflict of interest statement
Figures


References
-
- Warburton D, Berberich MA, Driscoll B. Stem/progenitor cells in lung morphogenesis, repair, and regeneration. Curr Top Dev Biol. 2004;64:1–16. - PubMed
-
- Hong KU, Reynolds SD, Giangreco A, et al. Clara cell secretory protein-expressing cells of the airway neuroepithelial body microenvironment include a label-retaining subset and are critical for epithelial renewal after progenitor cell depletion. Am J Respir Cell Mol Biol. 2001;24:671–681. - PubMed
-
- Kim CF, Jackson EL, Woolfenden AE, et al. Identification of bronchioalveolar stem cells in normal lung and lung cancer. Cell. 2005;121:823–835. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

