Downregulation of KSR1 in pancreatic cancer xenografts by antisense oligonucleotide correlates with tumor drug uptake
- PMID: 18719367
- PMCID: PMC2576298
- DOI: 10.4161/cbt.7.9.6472
Downregulation of KSR1 in pancreatic cancer xenografts by antisense oligonucleotide correlates with tumor drug uptake
Abstract
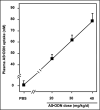
While antisense oligonucleotide (AS-ODN) technology holds promise for the treatment of cancer, to date there have been no clinical successes. Unfortunately, current assays are not sufficiently sensitive to measure tissue ODN levels. Hence it has not been possible to ascertain whether treatment failures result from failure of drug delivery. To investigate the relationship between drug uptake and therapeutic effect, we developed an ultrasensitive noncompetitive hybridization-ligation enzyme-linked immunosorbent assay (NCHL-ELISA) to quantify Kinase Suppressor of Ras1 (KSR1) AS-ODN drug uptake in plasma and tumor tissues. In mice harboring PANC-1 pancreatic cancer xenografts and continuously infused with AS-ODN, our ELISA detects plasma and tumor KSR1 AS-ODN levels over an extended range, from 0.05 nM to 20 nM. Using this sensitive assay, we demonstrate that KSR1 repression in pancreatic cancer xenografts correlates highly with AS-ODN uptake into tumor tissues. In contrast, plasma drug levels do not correlate with tumor drug content or target downregulation. These studies indicate the efficacy of our ELISA, and suggest that tumor biopsy material will need to be procured to estimate the potential of this antisense technology.
Figures



References
-
- Kornfeld K, Guan KL, Horvitz HR. The Caenorhabditis elegans gene mek-2 is required for vulval induction and encodes a protein similar to the protein kinase MEK. Genes Dev. 1995;9:756–68. - PubMed
-
- Kornfeld K, Hom DB, Horvitz HR. The ksr-1 gene encodes a novel protein kinase involved in Ras-mediated signaling in C. elegans. Cell. 1995;83:903–13. - PubMed
-
- Sundaram M, Han M. The C. elegans ksr-1 gene encodes a novel Raf-related kinase involved in Ras-mediated signal transduction. Cell. 1995;83:889–901. - PubMed
-
- Therrien M, Chang HC, Solomon NM, Karim FD, Wassarman DA, Rubin GM. KSR, a novel protein kinase required for RAS signal transduction. Cell. 1995;83:879–88. - PubMed
-
- Lozano J, Xing R, Cai Z, Jensen HL, Trempus C, Mark W, Cannon R, Kolesnick R. Deficiency of kinase suppressor of Ras1 prevents oncogenic ras signaling in mice. Cancer Res. 2003;63:4232–8. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
