Immune stimulatory antigen loaded particles combined with depletion of regulatory T-cells induce potent tumor specific immunity in a mouse model of melanoma
- PMID: 18719913
- PMCID: PMC11030463
- DOI: 10.1007/s00262-008-0574-6
Immune stimulatory antigen loaded particles combined with depletion of regulatory T-cells induce potent tumor specific immunity in a mouse model of melanoma
Abstract
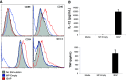
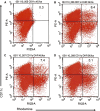
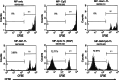
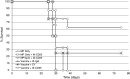
Anti-tumor vaccines capable of activating both CD4 and CD8 T cells are preferred for long lasting T cell responses. Induction of a tumor-specific T-cell response can be induced by tumor vaccines that target innate immunity. The ensuing T-cell response depends on efficient antigen presentation from phagocytosed cargo in the antigen presenting cell and is augmented by the presence of Toll-like receptor (TLR) ligands within the cargo. Biodegradable polymers are useful for vaccine delivery in that they are phagocytosed by antigen presenting cells (APCs) and could potentially be loaded with both the antigen and immune stimulatory TLR agents. This study was undertaken to evaluate the effect of poly lactic-co-glycolic acid (PLGA) polymer particles loaded with antigenic tumor lysate and immune stimulatory CpG oligonucleotides on induction of tumor specific immunity in a mouse model of melanoma. We found that after delivery, these immune stimulatory antigen loaded particles (ISAPs) efficiently activated APCs and were incorporated into lysosomal compartments of macrophages and dendritic cells. ISAP vaccination resulted in remarkable T cell proliferation, but only modestly suppressed tumor growth of established melanoma. Due to this discordant effect on tumor immunity we evaluated the role of regulatory T cells (Treg) and found that ISAP vaccination or tumor growth alone induced prolific expansion of tumor specific Treg. When the Treg compartment was suppressed with anti-CD25 antibody, ISAP vaccination induced complete antigen-specific immunity in a prophylactic model. ISAP vaccination is a novel tumor vaccine strategy that is designed to co-load the antigen with a TLR agonist enabling efficient Ag presentation. Targeting of T-reg expansion during vaccination may be necessary for inducing effective tumor-specific immunity.
Figures





References
-
- Steinman RM. Linking innate to adaptive immunity through dendritic cells. Novartis Found Symp. 2006;279:101–109. - PubMed
-
- Krieg AM, Efler SM, Wittpoth M, Al Adhami MJ, Davis HL. Induction of systemic TH1-like innate immunity in normal volunteers following subcutaneous but not intravenous administration of CPG 7909, a synthetic B-class CpG oligodeoxynucleotide TLR9 agonist. J Immunother. 2004;27:460–471. doi: 10.1097/00002371-200411000-00006. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

