Neutrophil recruitment under shear flow: it's all about endothelial cell rings and gaps
- PMID: 18720226
- PMCID: PMC2726622
- DOI: 10.1080/10739680802273892
Neutrophil recruitment under shear flow: it's all about endothelial cell rings and gaps
Erratum in
- Microcirculation. 2009 Nov;16(8):782
Abstract
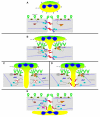
Leukocyte recruitment to tissues and organs is an essential component of host defense. The molecular mechanisms controlling this process are complex and remain under active investigation. The combination of biochemical techniques and live cell imaging using in vivo and in vitro flow-model approaches have shed light on several aspects of neutrophil transmigration through the vascular endothelial lining of blood vessels. Here, we focus on the role of adhesion molecule signaling in endothelial cells and their downstream targets during the process of transendothelial migration at cell-cell borders (paracellular transmigration). An emerging model involves the leukocyte beta2 integrin engagement of endothelial cell ICAM-1, which triggers integrin-ICAM-1 clustering (rings) and stabilizes leukocyte adhesion at cell-cell junctions. This step recruits nonreceptor tyrosine kinases that phosphorylate key tyrosine residues in the cytoplasmic tail of VE-cadherin, which destabilizes its linkage to catenins and the actin cytoskeleton, triggering the transient opening of VE-cadherin homodimers to form a gap in the cell junction, through which the neutrophil transmigrates. Interestingly, the signaling events that lead to neutrophil transmigration occur independently of shear flow in vitro.
Figures


References
-
- Adamson P, Etienne S, Couraud PO, Calder V, Greenwood J. Lymphocyte migration through brain endothelial cell monolayers involves signaling through endothelial ICAM-1 via a rho-dependent pathway. J Immunol. 1999;162:2964–2973. - PubMed
-
- Allingham MJ, van Buul JD, Burridge K. ICAM-1-Mediated, Src- and Pyk2-Dependent Vascular Endothelial Cadherin Tyrosine Phosphorylation Is Required for Leukocyte Transendothelial Migration. J Immunol. 2007;179:4053–4064. - PubMed
-
- Allport JR, Ding H, Collins T, Gerritsen ME, Luscinskas FW. Endothelial-dependent mechanisms regulate leukocyte transmigration: A process involving the proteasome and disruption of the vascular endothelial-cadherin complex at endothelial cell-to-cell junctions. J Exp Med. 1997;186:517–527. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

