Prevention of hepatotoxicity due to anti tuberculosis treatment: a novel integrative approach
- PMID: 18720535
- PMCID: PMC2739336
- DOI: 10.3748/wjg.14.4753
Prevention of hepatotoxicity due to anti tuberculosis treatment: a novel integrative approach
Abstract
Aim: To evaluate the ability of Curcuma longa (CL) and Tinospora cordifolia (TC) formulation to prevent anti-tuberculosis (TB) treatment (ATT) induced hepatotoxicity.
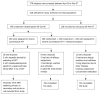
Methods: Patients with active TB diagnosis were randomized to a drug control group and a trial group on drugs plus an herbal formulation. Isoniazid, rifampicin, pyrazinamide and ethambutol for first 2 mo followed by continuation phase therapy excluding Pyrazinamide for 4 mo comprised the anti-tuberculous treatment. Curcumin enriched (25%) CL and a hydro-ethanolic extract enriched (50%) TC 1 g each divided in two doses comprised the herbal adjuvant. Hemogram, bilirubin and liver enzymes were tested initially and monthly till the end of study to evaluate the result.
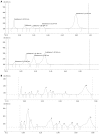
Results: Incidence and severity of hepatotoxicity was significantly lower in trial group (incidence: 27/192 vs 2/316, P<0.0001). Mean aspartate transaminase (AST) (195.93+/-108.74 vs 85+/-4.24, P<0.0001), alanine transaminase (ALT) (75.74+/-26.54 vs 41+/-1.41, P<0.0001) and serum bilirubin (5.4+/-3.38 vs 1.5+/-0.42, P<0.0001). A lesser sputum positivity ratio at the end of 4 wk (10/67 vs 4/137, P=0.0068) and decreased incidence of poorly resolved parenchymal lesion at the end of the treatment (9/152 vs 2/278, P=0.0037) was observed. Improved patient compliance was indicated by nil drop-out in trial vs 10/192 in control group (P<0.0001).
Conclusion: The herbal formulation prevented hepatotoxicity significantly and improved the disease outcome as well as patient compliance without any toxicity or side effects.
Figures
References
-
- Runyon EH. Preventive Treatment in Tuberculosis: A Statement by the Committee on Therapy. American Thoracic Society. Am Rev Respir Dis. 1965;91:297–298. - PubMed
-
- Garibaldi RA, Drusin RE, Ferebee SH, Gregg MB. Isoniazid-associated hepatitis. Report of an outbreak. Am Rev Respir Dis. 1972;106:357–365. - PubMed
-
- Kopanoff DE, Snider DE Jr, Caras GJ. Isoniazid-related hepatitis: a U.S. Public Health Service cooperative surveillance study. Am Rev Respir Dis. 1978;117:991–1001. - PubMed
-
- Steele MA, Burk RF, DesPrez RM. Toxic hepatitis with isoniazid and rifampin. A meta-analysis. Chest. 1991;99:465–471. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

