Lysine acetylation: codified crosstalk with other posttranslational modifications
- PMID: 18722172
- PMCID: PMC2551738
- DOI: 10.1016/j.molcel.2008.07.002
Lysine acetylation: codified crosstalk with other posttranslational modifications
Abstract
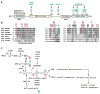
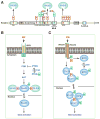
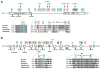
Lysine acetylation has emerged as a major posttranslational modification for histones. Crossregulation between this and other modifications is crucial in modulating chromatin-based transcriptional control and shaping inheritable epigenetic programs. In addition to histones, many other nuclear proteins and various cytoplasmic regulators are subject to lysine acetylation. This review focuses on recent findings pertinent to acetylation of nonhistone proteins and emphasizes how this modification might crosstalk with phosphorylation, methylation, ubiquitination, sumoylation, and others to form code-like multisite modification programs for dynamic control of cellular signaling under diverse conditions.
Figures



References
-
- Allis CD, Berger SL, Cote J, Dent S, Jenuwien T, Kouzarides T, Pillus L, Reinberg D, Shi Y, Shiekhattar R, et al. New nomenclature for chromatin-modifying enzymes. Cell. 2007;131:633–636. - PubMed
-
- Appella E, Anderson CW. Signaling to p53: breaking the posttranslational modification code. Pathol Biol (Paris) 2000;48:227–245. - PubMed
-
- Bali P, Pranpat M, Bradner J, Balasis M, Fiskus W, Guo F, Rocha K, Kumaraswamy S, Boyapalle S, Atadja P, et al. Inhibition of histone deacetylase 6 acetylates and disrupts the chaperone function of heat shock protein 90: a novel basis for antileukemia activity of histone deacetylase inhibitors. J Biol Chem. 2005;280:26729–26734. - PubMed
-
- Berger SL. The complex language of chromatin regulation during transcription. Nature. 2007;447:407–412. - PubMed
-
- Bhaumik SR, Smith E, Shilatifard A. Covalent modifications of histones during development and disease pathogenesis. Nat Struct Mol Biol. 2007;14:1008–1016. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

