Organization and evolution of the biological response to singlet oxygen stress
- PMID: 18723027
- PMCID: PMC2579311
- DOI: 10.1016/j.jmb.2008.08.017
Organization and evolution of the biological response to singlet oxygen stress
Abstract
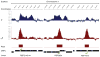
The appearance of atmospheric oxygen from photosynthetic activity led to the evolution of aerobic respiration and responses to the resulting reactive oxygen species. In Rhodobacter sphaeroides, a photosynthetic alpha-proteobacterium, a transcriptional response to the reactive oxygen species singlet oxygen ((1)O(2)) is controlled by the group IV sigma factor sigma(E) and the anti-sigma factor ChrR. In this study, we integrated various large datasets to identify genes within the (1)O(2) stress response that contain sigma(E)-dependent promoters both within R. sphaeroides and across the bacterial phylogeny. Transcript pattern clustering and a sigma(E)-binding sequence model were used to predict candidate promoters that respond to (1)O(2) stress in R. sphaeroides. These candidate promoters were experimentally validated to nine R. sphaeroides sigma(E)-dependent promoters that control the transcription of 15 (1)O(2)-activated genes. Knowledge of the R. sphaeroides response to (1)O(2) and its regulator sigma(E)-ChrR was combined with large-scale phylogenetic and sequence analyses to predict the existence of a core set of approximately eight conserved sigma(E)-dependent genes in alpha-proteobacteria and gamma-proteobacteria. The bacteria predicted to contain this conserved response to (1)O(2) include photosynthetic species, as well as free-living and symbiotic/pathogenic nonphotosynthetic species. Our analysis also predicts that the response to (1)O(2) evolved within the time frame of the accumulation of atmospheric molecular oxygen on this planet.
Figures

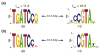



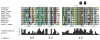

References
-
- Kotelnikova EA, Makeev VJ, Gelfand MS. Evolution of transcription factor DNA binding sites. Gene. 2005;347:255–263. - PubMed
-
- Koonin EV. Orthologs, paralogs, and evolutionary genomics. Annu Rev Genet. 2005;39:309–338. - PubMed
-
- Madan Babu M, Teichmann SA, Aravind L. Evolutionary dynamics of prokaryotic transcriptional regulatory networks. J Mol Biol. 2006;358:614–633. - PubMed
-
- Mackenzie C, Eraso JM, Choudhary M, Roh JH, Zeng X, Bruscella P, et al. Postgenomic adventures with Rhodobacter sphaeroides. Annu Rev Microbiol. 2007;61:283–307. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

