Rapidly increased neuronal mitochondrial biogenesis after hypoxic-ischemic brain injury
- PMID: 18723421
- PMCID: PMC2726706
- DOI: 10.1161/STROKEAHA.108.520114
Rapidly increased neuronal mitochondrial biogenesis after hypoxic-ischemic brain injury
Abstract
Background and purpose: Mitochondrial biogenesis is regulated through the coordinated actions of both nuclear and mitochondrial genomes to ensure that the organelles are replenished on a regular basis. This highly regulated process has been well defined in skeletal and heart muscle, but its role in neuronal cells, particularly when under stress or injury, is not well understood. In this study, we report for the first time rapidly increased mitochondrial biogenesis in a rat model of neonatal hypoxic/ischemic brain injury (H-I).
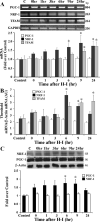
Methods: Postnatal day 7 rats were subjected to H-I induced by unilateral carotid artery ligation followed by 2.5 hours of hypoxia. The relative amount of brain mitochondrial DNA (mtDNA) was measured semiquantitatively using long fragment PCR at various time points after H-I. HSP60 and COXIV proteins were detected by Western blot. Expression of three genes critical for the transcriptional regulation of mitochondrial biogenesis, peroxisome proliferator-activated receptor coactivator-1 (PGC-1), nuclear respiratory factor-1 (NRF-1), and mitochondrial transcription factor A (TFAM), were examined by Western blot and RT-PCR.
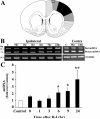
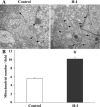
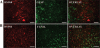
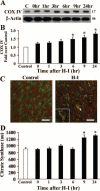
Results: Brain mtDNA content was markedly increased 6 hours after H-I, and continued to increase up to 24 hours after H-I. Paralleling the temporal change in mtDNA content, mitochondrial number and proteins HSP60 and COXIV, and citrate synthase activity were increased in neurons in the cortical infarct border zone after H-I. Moreover, cortical expression of NRF-1 and TFAM were increased 6 to 24 hours after H-I, whereas PGC-1 was not changed.
Conclusions: Neonatal H-I brain injury rapidly induces mitochondrial biogenesis, which may constitute a novel component of the endogenous repair mechanisms of the brain.
Figures

References
-
- Fiskum G. Mitochondrial participation in ischemic and traumatic neural cell death. J Neurotraum. 2000;17:843–855. - PubMed
-
- St-Pierre J, Drori S, Uldry M, Silvaggi JM, Rhee J, Jager S, Handschin C, Zheng K, Lin J, Yang W, Simon DK, Bachoo R, Spiegelman BM. Suppression of reactive oxygen species and neurodegeneration by the pgc-1 transcriptional coactivators. Cell. 2006;127:397–408. - PubMed
-
- Scarpulla RC. Nuclear control of respiratory gene expression in mammalian cells. J of Cell Biochem. 2006;97:673–683. - PubMed
-
- Blomgren K, Hagberg H. Free radicals, mitochondria, and hypoxia-ischemia in the developing brain. Free Radic Biol Med. 2006;40:388–397. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

