Structural classification of toxic amyloid oligomers
- PMID: 18723507
- PMCID: PMC2573087
- DOI: 10.1074/jbc.R800016200
Structural classification of toxic amyloid oligomers
Abstract
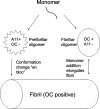
Amyloid oligomers are believed to play important causal roles in many types of amyloid-related degenerative diseases. Many different laboratories have reported amyloid oligomers that differ in size, morphology, toxicity, and method of preparation or purification, raising the question of the structural relationships among these oligomer preparations. The structural plasticity that has been reported to occur in amyloids formed from the same protein sequence indicates that it is quite possible that different oligomer preparations may represent distinct structural variants. In view of the difficulty in determining the precise structure of amyloids, conformation- and epitope-specific antibodies may provide a facile means of classifying amyloid oligomer structures. Conformation-dependent antibodies that recognize generic epitopes that are specifically associated with distinct aggregation states of many different amyloid-forming sequences indicate that there are at least two fundamentally distinct types of amyloid oligomers: fibrillar and prefibrillar oligomers. Classification of amyloid oligomers according to their underlying structures may be a more useful and rational approach than relying on differences in size and morphology.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

