Use of CDP-glycerol as an alternate acceptor for the teichoic acid polymerase reveals that membrane association regulates polymer length
- PMID: 18723614
- PMCID: PMC2580681
- DOI: 10.1128/JB.00851-08
Use of CDP-glycerol as an alternate acceptor for the teichoic acid polymerase reveals that membrane association regulates polymer length
Abstract
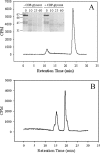
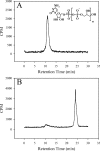
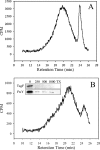
The study of bacterial extracellular polysaccharide biosynthesis is hampered by the fact that these molecules are synthesized on membrane-resident carrier lipids. To get around this problem, a practical solution has been to synthesize soluble lipid analogs and study the biosynthetic enzymes using a soluble system. This has been done for the Bacillus subtilis teichoic acid polymerase, TagF, although several aspects of catalysis were inconsistent with the results obtained with reconstituted membrane systems or physiological observations. In this work we explored the acceptor substrate promiscuity and polymer length disregulation that appear to be characteristic of TagF activity away from biological membranes. Using isotope labeling, steady-state kinetics, and chemical lability studies, we demonstrated that the enzyme can synthesize poly(glycerol phosphate) teichoic acid using the elongation substrate CDP-glycerol as an acceptor. This suggests that substrate specificity is relaxed in the region distal to the glycerol phosphate moiety in the acceptor molecule under these conditions. Polymer synthesis proceeded at a rate (27 min(-1)) comparable to that in the reconstituted membrane system after a distinct lag period which likely represented slower initiation on the unnatural CDP-glycerol acceptor. We confirmed that polymer length became disregulated in the soluble system as the polymers synthesized on CDP-glycerol acceptors were much larger than the polymers synthesized on the membrane or previously found attached to bacterial cell walls. Finally, polymer synthesis on protease-treated membranes suggested that proper length regulation is retained in the absence of accessory proteins and provided evidence that such regulation is conferred through proper association of the polymerase with the membrane.
Figures



Similar articles
-
Structure of the bacterial teichoic acid polymerase TagF provides insights into membrane association and catalysis.Nat Struct Mol Biol. 2010 May;17(5):582-9. doi: 10.1038/nsmb.1819. Epub 2010 Apr 18. Nat Struct Mol Biol. 2010. PMID: 20400947
-
Purified, recombinant TagF protein from Bacillus subtilis 168 catalyzes the polymerization of glycerol phosphate onto a membrane acceptor in vitro.J Biol Chem. 2003 May 16;278(20):18002-7. doi: 10.1074/jbc.M300706200. Epub 2003 Mar 12. J Biol Chem. 2003. PMID: 12637499
-
The wall teichoic acid polymerase TagF is non-processive in vitro and amenable to study using steady state kinetic analysis.J Biol Chem. 2009 Aug 7;284(32):21132-8. doi: 10.1074/jbc.M109.010215. Epub 2009 Jun 11. J Biol Chem. 2009. PMID: 19520862 Free PMC article.
-
Occurrence and function of membrane teichoic acids.Biochim Biophys Acta. 1977 May 31;472(1):1-12. doi: 10.1016/0304-4157(77)90012-0. Biochim Biophys Acta. 1977. PMID: 406922 Review.
-
Biosynthesis and Export of Bacterial Glycolipids.Annu Rev Biochem. 2020 Jun 20;89:741-768. doi: 10.1146/annurev-biochem-011520-104707. Annu Rev Biochem. 2020. PMID: 32569526 Review.
Cited by
-
ABC transporters involved in export of cell surface glycoconjugates.Microbiol Mol Biol Rev. 2010 Sep;74(3):341-62. doi: 10.1128/MMBR.00009-10. Microbiol Mol Biol Rev. 2010. PMID: 20805402 Free PMC article. Review.
-
Characterization of Wall Teichoic Acid Degradation by the Bacteriophage ϕ29 Appendage Protein GP12 Using Synthetic Substrate Analogs.J Biol Chem. 2015 Jul 31;290(31):19133-45. doi: 10.1074/jbc.M115.662866. Epub 2015 Jun 17. J Biol Chem. 2015. PMID: 26085106 Free PMC article.
-
Wall teichoic acids of gram-positive bacteria.Annu Rev Microbiol. 2013;67:313-36. doi: 10.1146/annurev-micro-092412-155620. Annu Rev Microbiol. 2013. PMID: 24024634 Free PMC article. Review.
-
Structure of the bacterial teichoic acid polymerase TagF provides insights into membrane association and catalysis.Nat Struct Mol Biol. 2010 May;17(5):582-9. doi: 10.1038/nsmb.1819. Epub 2010 Apr 18. Nat Struct Mol Biol. 2010. PMID: 20400947
-
Design, synthesis and optimization of TarO inhibitors as multifunctional antibiotics against Methicillin-resistant Staphylococcus aureus.NPJ Antimicrob Resist. 2025 Apr 12;3(1):28. doi: 10.1038/s44259-025-00098-z. NPJ Antimicrob Resist. 2025. PMID: 40221595 Free PMC article.
References
-
- Allinquant, B., C. Musenger, and E. Schuller. 1985. Reversed-phase high-performance liquid chromatography of nucleotides and oligonucleotides. J. Chromatogr. 326281-291.
-
- Badurina, D. S. 2003. CTP:glycerol 3-phosphate cytidylyltransferase (TarD) from Staphylococcus aureus catalyzes the cytidylyl transfer via an ordered Bi-Bi reaction mechanism with micromolar Km values. Biochim. Biophys. Acta 1646196-206. - PubMed
-
- Bastin, D. A., G. Stevenson, P. K. Brown, A. Haase, and P. R. Reeves. 1993. Repeat unit polysaccharides of bacteria: a model for polymerization resembling that of ribosomes and fatty acid synthetase, with a novel mechanism for determining chain length. Mol. Microbiol. 7725-734. - PubMed
-
- Bhavsar, A. P., R. Truant, and E. D. Brown. 2005. The TagB protein in Bacillus subtilis 168 is an intracellular peripheral membrane protein that can incorporate glycerol phosphate onto a membrane-bound acceptor in vitro. J. Biol. Chem. 28036691-36700. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

