Reexamination of the role of the amino terminus of SecA in promoting its dimerization and functional state
- PMID: 18723626
- PMCID: PMC2580686
- DOI: 10.1128/JB.00593-08
Reexamination of the role of the amino terminus of SecA in promoting its dimerization and functional state
Abstract
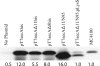
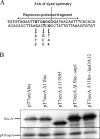
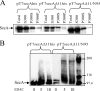
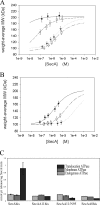
The SecA nanomotor promotes protein translocation in eubacteria by binding both protein cargo and the protein-conducting channel and by undergoing ATP-driven conformation cycles that drive this process. There are conflicting reports about whether SecA functions as a monomer or dimer during this dynamic process. Here we reexamined the roles of the amino and carboxyl termini of SecA in promoting its dimerization and functional state by examining three secA mutants and the corresponding proteins: SecADelta8 lacking residues 2 to 8, SecADelta11 lacking residues 2 to 11, and SecADelta11/N95 lacking both residues 2 to 11 and the carboxyl-terminal 70 residues. We demonstrated that whether SecADelta11 or SecADelta11/N95 was functional for promoting cell growth depended solely on the vivo level of the protein, which appeared to govern residual dimerization. All three SecA mutant proteins were defective for promoting cell growth unless they were highly overproduced. Cell fractionation revealed that SecADelta11 and SecADelta11/N95 were proficient in membrane association, although the formation of integral membrane SecA was reduced. The presence of a modestly higher level of SecADelta11/N95 in the membrane and the ability of this protein to form dimers, as detected by chemical cross-linking, were consistent with the higher level of secA expression and better growth of the SecADelta11/N95 mutant than of the SecADelta11 mutant. Biochemical studies showed that SecADelta11 and SecADelta11/N95 had identical dimerization defects, while SecADelta8 was intermediate between these proteins and wild-type SecA in terms of dimer formation. Furthermore, both SecADelta11 and SecADelta11/N95 were equally defective in translocation ATPase specific activity. Our studies showed that the nonessential carboxyl-terminal 70 residues of SecA play no role in its dimerization, while increasing the truncation of the amino-terminal region of SecA from 8 to 11 residues results in increased defects in SecA dimerization and poor in vivo function unless the protein is highly overexpressed. They also clarified a number of conflicting previous reports and support the essential nature of the SecA dimer.
Figures
References
-
- Barkley, M. D., and S. Bourgeois. 1980. Repressor recognition of operator and effectors, p. 177-220. In J. H. Miller and W. S. Reznikoff (ed.), The operon, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
-
- Benach, J., Y.-T. Chou, J. J. Fak, A. Itkin, D. D. Nicolae, P. C. Smith, G. Wittrock, D. L. Floyd, C. M. Golsaz, L. M. Gierasch, and J. F. Hunt. 2003. Phospholipid-induced monomerization and signal-peptide-induced oligomerization of SecA. J. Biol. Chem. 2783628-3638. - PubMed
-
- Breukink, E., N. Nouwen, A. van Raalte, S. Mizushima, J. Tommassen, and B. de Kruijff. 1995. The C terminus of SecA is involved in both lipid binding and SecB binding. J. Biol. Chem. 270:7902-7907. - PubMed
-
- Cabelli, R. J. 1991. Biochemical characterization of the role of SecA protein in protein export in Escherichia coli. Ph.D. thesis. State University of New York, Stony Brook, NY.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

