RPA phosphorylation facilitates mitotic exit in response to mitotic DNA damage
- PMID: 18723675
- PMCID: PMC2529075
- DOI: 10.1073/pnas.0803001105
RPA phosphorylation facilitates mitotic exit in response to mitotic DNA damage
Abstract
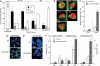
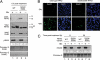
Human replication protein A (RPA) becomes phosphorylated on the RPA2 subunit by cyclin B-Cdc2 during mitosis, although the functional role of this modification is unclear. We find that this modification stimulates RPA2 to become hyperphosphorylated in response to mitotic DNA damage caused by bleomycin treatment. Cells in which endogenous RPA2 was replaced by a mutant subunit lacking both Cdc2 sites had a significant defect in mitotic release into a 2N G(1) phase after exposure to bleomycin. An increased percentage of these mutant cells also was positive initially for cyclin B expression and BubR1 chromatin staining, indicative of an extended spindle assembly checkpoint. The mutant cells that experienced mitotic DNA damage also underwent apoptosis at higher levels than cells expressing the WT subunit. Even so, we did not find the mutation had any dramatic effects on the level of DNA repair in mitosis. Cells lacking ATM (a checkpoint factor and RPA2 kinase) also were severely defective in mitotic exit and were unable to support RPA hyperphosphorylation after mitotic DNA damage. Although checkpoint 1 effector kinase (Chk1) had a more complex role, inhibition of Chk1 activity with UCN-01 also reduced mitotic exit. Chk1 activation and mitotic RPA hyperphosphorylation were found to be independent events. Our results demonstrate that mitotic RPA hyperphosphorylation facilitates release of cells from a damaged mitosis into a 2N G(1) phase, thereby increasing cell viability.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
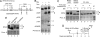
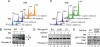
Similar articles
-
Mitotic crisis: the unmasking of a novel role for RPA.Cell Cycle. 2009 Feb 1;8(3):357-61. doi: 10.4161/cc.8.3.7496. Epub 2009 Feb 21. Cell Cycle. 2009. PMID: 19176996 Free PMC article. Review.
-
Distinct roles for DNA-PK, ATM and ATR in RPA phosphorylation and checkpoint activation in response to replication stress.Nucleic Acids Res. 2012 Nov;40(21):10780-94. doi: 10.1093/nar/gks849. Epub 2012 Sep 12. Nucleic Acids Res. 2012. PMID: 22977173 Free PMC article.
-
Ionizing radiation-dependent and independent phosphorylation of the 32-kDa subunit of replication protein A during mitosis.Nucleic Acids Res. 2009 Oct;37(18):6028-41. doi: 10.1093/nar/gkp605. Epub 2009 Aug 11. Nucleic Acids Res. 2009. PMID: 19671522 Free PMC article.
-
Sequential and synergistic modification of human RPA stimulates chromosomal DNA repair.J Biol Chem. 2007 Dec 7;282(49):35910-23. doi: 10.1074/jbc.M704645200. Epub 2007 Oct 10. J Biol Chem. 2007. PMID: 17928296
-
Replication checkpoint: preventing mitotic catastrophe.Curr Biol. 2001 Feb 20;11(4):R121-4. doi: 10.1016/s0960-9822(01)00057-4. Curr Biol. 2001. PMID: 11250164 Review.
Cited by
-
N terminus of CtIP is critical for homologous recombination-mediated double-strand break repair.J Biol Chem. 2009 Nov 13;284(46):31746-52. doi: 10.1074/jbc.M109.023424. Epub 2009 Sep 16. J Biol Chem. 2009. PMID: 19759395 Free PMC article.
-
Involvement of nucleotide excision and mismatch repair mechanisms in double strand break repair.Curr Genomics. 2009 Jun;10(4):250-8. doi: 10.2174/138920209788488544. Curr Genomics. 2009. PMID: 19949546 Free PMC article.
-
DNA damage response is suppressed by the high cyclin-dependent kinase 1 activity in mitotic mammalian cells.J Biol Chem. 2011 Oct 14;286(41):35899-35905. doi: 10.1074/jbc.M111.267690. Epub 2011 Aug 30. J Biol Chem. 2011. PMID: 21878640 Free PMC article.
-
Mitotic crisis: the unmasking of a novel role for RPA.Cell Cycle. 2009 Feb 1;8(3):357-61. doi: 10.4161/cc.8.3.7496. Epub 2009 Feb 21. Cell Cycle. 2009. PMID: 19176996 Free PMC article. Review.
-
Managing Single-Stranded DNA during Replication Stress in Fission Yeast.Biomolecules. 2015 Sep 18;5(3):2123-39. doi: 10.3390/biom5032123. Biomolecules. 2015. PMID: 26393661 Free PMC article. Review.
References
-
- Sanchez Y, et al. Control of the DNA damage checkpoint by chk1 and rad53 protein kinases through distinct mechanisms. Science. 1999;286:1166–1171. - PubMed
-
- Skoufias DA, Lacroix FB, Andreassen PR, Wilson L, Margolis RL. Inhibition of DNA decatenation, but not DNA damage, arrests cells at metaphase. Mol Cell. 2004;15:977–990. - PubMed
-
- Iftode C, Daniely Y, Borowiec JA. Replication protein A (RPA): The eukaryotic SSB. Crit Rev Biochem Mol Biol. 1999;34:141–180. - PubMed
-
- Binz SK, Sheehan AM, Wold MS. Replication protein A phosphorylation and the cellular response to DNA damage. DNA Repair (Amst) 2004;3:1015–1024. - PubMed
-
- Zou L, Elledge SJ. Sensing DNA damage through ATRIP recognition of RPA-ssDNA complexes. Science. 2003;300:1542–1548. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

