Androgen receptor is a tumor suppressor and proliferator in prostate cancer
- PMID: 18723679
- PMCID: PMC2527886
- DOI: 10.1073/pnas.0804700105
Androgen receptor is a tumor suppressor and proliferator in prostate cancer
Abstract
Targeting androgens/androgen receptor (AR) functions via androgen deprivation therapy (ADT) remains the standard treatment for prostate cancer. However, most tumors eventually recur despite ADT. Here we demonstrate that the prostate AR may function as both a suppressor and a proliferator to suppress or promote prostate cancer metastasis. Results from orthotopically recombining stromal WPMY1 cells with epithelial PC3 prostate cancer cells in mice demonstrated that restoring AR in epithelial PC3 cells or knockdown of AR in stromal WPMY1 cells suppressed prostate cancer metastasis. Knockdown of the AR in epithelial CWR22rv1 prostate cancer cells also resulted in increased cell invasion in vitro and in vivo. Restoring AR in PC3 cells (PC3-AR9) results in decreased invasion in bone lesion assays and in vivo mouse models. Mice lacking the prostate epithelial AR have increased apoptosis in epithelial luminal cells and increased proliferation in epithelial basal cells. The consequences of these two contrasting results led to the expansion of CK5/CK8-positive intermediate cells, and mice developed larger and more invasive metastatic tumors in lymph nodes and died earlier than wild-type littermates. Mechanistic dissection suggested that androgens/AR might directly or indirectly modulate metastasis-related genes and suppression of TGFbeta1 signals results in the partial inhibition of AR-mediated metastasis. Collectively, our understanding of these opposing roles of prostatic AR may revolutionize the way we combat prostate cancer, and allow the development of new and better therapies by targeting only the proliferative role of AR.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


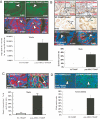
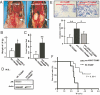
References
-
- Chang CS, Kokontis J, Liao ST. Molecular cloning of human and rat complementary DNA encoding androgen receptors. Science. 1988;240:324–326. - PubMed
-
- Heinlein CA, Chang C. Androgen receptor in prostate cancer. Endocr Rev. 2004;25:276–308. - PubMed
-
- Miyamoto H, Messing EM, Chang C. Does androgen deprivation improve treatment outcomes in patients with low-risk and intermediate-risk prostate cancer? Nat Clin Pract Oncol. 2005;2:236–237. - PubMed
-
- Fluchter SH, Weiser R, Gamper C. The role of hormonal treatment in prostate cancer. Recent Results Cancer Res. 2007;175:211–237. - PubMed
-
- Mizokami A, Yeh SY, Chang C. Identification of 3′,5′-cyclic adenosine monophosphate response element and other cis-acting elements in the human androgen receptor gene promoter. Mol Endocrinol. 1994;8:77–88. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

