AICAR decreases the activity of two distinct amiloride-sensitive Na+-permeable channels in H441 human lung epithelial cell monolayers
- PMID: 18723760
- PMCID: PMC2584878
- DOI: 10.1152/ajplung.90353.2008
AICAR decreases the activity of two distinct amiloride-sensitive Na+-permeable channels in H441 human lung epithelial cell monolayers
Abstract
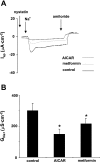
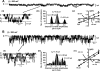
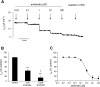
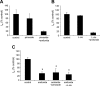
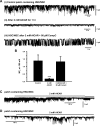
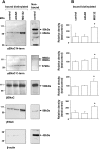
Transepithelial transport of Na(+) across the lung epithelium via amiloride-sensitive Na(+) channels (ENaC) regulates fluid volume in the lung lumen. Activators of AMP-activated protein kinase (AMPK), the adenosine monophosphate mimetic AICAR, and the biguanide metformin decreased amiloride-sensitive apical Na(+) conductance (G(Na+)) in human H441 airway epithelial cell monolayers. Cell-attached patch-clamp recordings identified two distinct constitutively active cation channels in the apical membrane that were likely to contribute to G(Na+): a 5-pS highly Na(+) selective ENaC-like channel (HSC) and an 18-pS nonselective cation channel (NSC). Substituting NaCl with NMDG-Cl in the patch pipette solution shifted the reversal potentials of HSC and NSC, respectively, from +23 mV to -38 mV and 0 mV to -35 mV. Amiloride at 1 microM inhibited HSC activity and 56% of short-circuit current (I(sc)), whereas 10 microM amiloride partially reduced NSC activity and inhibited a further 30% of I(sc). Neither conductance was associated with CNG channels as there was no effect of 10 microM pimoside on I(sc), HSC, or NSC activity, and 8-bromo-cGMP (0.3-0.1 mM) did not induce or increase HSC or NSC activity. Pretreatment of H441 monolayers with 2 mM AICAR inhibited HSC/NSC activity by 90%, and this effect was reversed by the AMPK inhibitor Compound C. All three ENaC proteins were identified in the apical membrane of H441 monolayers, but no change in their abundance was detected after treatment with AICAR. In conclusion, activation of AMPK with AICAR in H441 cell monolayers is associated with inhibition of two distinct amiloride-sensitive Na(+)-permeable channels by a mechanism that likely reduces channel open probability.
Figures


References
-
- Andreasen D, Vuagniaux G, Fowler-Jaeger N, Hummler E, Rossier BC. Activation of epithelial sodium channels by mouse channel activating proteases (mCAP) expressed in Xenopus oocytes requires catalytic activity of mCAP3 and mCAP2 but not mCAP1. J Am Soc Nephrol 17: 968–976, 2006. - PubMed
-
- Bals R, Beisswenger C, Blouquit S, Chinet T. Isolation and air-liquid interface culture of human large airway and bronchiolar epithelial cells. J Cyst Fibros 3, Suppl 2: 49–51, 2004. - PubMed
-
- Barry PH JPCalc, a software package for calculating liquid junction potential corrections in patch-clamp, intracellular, epithelial and bilayer measurements and for correcting junction potential measurements. J Neurosci Methods 51: 107–116, 1994. - PubMed
-
- Benos DJ, Awayda MS, Berdiev DK, Bradford AL, Fuller CM, Senyk O, Ismailov II. Diversity and regulation of amiloride-sensitive Na+ channels. Kidney Int 49: 1632–1637, 1996. - PubMed
-
- Bhalla V, Oyster NM, Fitch AC, Wijngaarden MA, Neumann D, Schlattner U, Pearce D, Hallows KR. AMP-activated kinase inhibits the epithelial Na+ channel through functional regulation of the ubiquitin ligase Nedd4-2. J Biol Chem 281: 26159–26169, 2006. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

