What is causing the reduced drug-placebo difference in recent schizophrenia clinical trials and what can be done about it?
- PMID: 18723840
- PMCID: PMC2879679
- DOI: 10.1093/schbul/sbn110
What is causing the reduced drug-placebo difference in recent schizophrenia clinical trials and what can be done about it?
Abstract
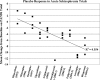
On September 18, 2007, a collaborative session between the International Society for CNS Clinical Trials and Methodology and the International Society for CNS Drug Development was held in Brussels, Belgium. Both groups, with membership from industry, academia, and governmental and nongovernmental agencies, have been formed to address scientific, clinical, regulatory, and methodological challenges in the development of central nervous system therapeutic agents. The focus of this joint session was the apparent diminution of drug-placebo differences in recent multicenter trials of antipsychotic medications for schizophrenia. To characterize the nature of the problem, some presenters reported data from several recent trials that indicated higher rates of placebo response and lower rates of drug response (even to previously established, comparator drugs), when compared with earlier trials. As a means to identify the possible causes of the problem, discussions covered a range of methodological factors such as participant characteristics, trial designs, site characteristics, clinical setting (inpatient vs outpatient), inclusion/exclusion criteria, and diagnostic specificity. Finally, possible solutions were discussed, such as improving precision of participant selection criteria, improving assessment instruments and/or assessment methodology to increase reliability of outcome measures, innovative methods to encourage greater subject adherence and investigator involvement, improved rater training and accountability metrics at clinical sites to increase quality assurance, and advanced methods of pharmacokinetic/pharmacodynamic modeling to optimize dosing prior to initiating large phase 3 trials. The session closed with a roundtable discussion and recommendations for data sharing to further explore potential causes and viable solutions to be applied in future trials.
Figures
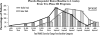
References
-
- Pangalos MN, Gallen CC. Drug discovery for disorders of the central nervous system. Neurotherapeutics. 2005;2:539–540.
-
- Kay SR, Fiszbein A, Opler LA. The Positive and Negative Syndrome Scale for schizophrenia. Schizophr Bull. 1987;13:261–276. - PubMed
-
- Faries DE, Heiligenstein JH, Tollefson GD, Potter WZ. The double blind variable placebo lead-in period: results from two antidepressant clinical trials. J Clin Psychopharmacol. 2001;21:561–568. - PubMed
-
- Mallinckrodt CH, Sanger TM, Dube S, et al. Assessing and interpreting treatment effects in longitudinal clinical trials with missing data. Biol Psychiatry. 2003;53:754–760. - PubMed

